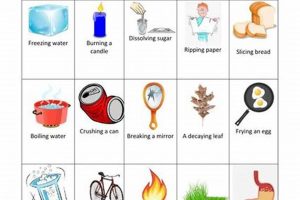
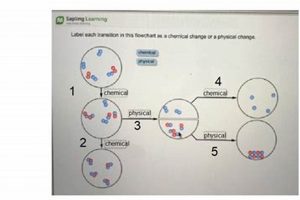
Laboratory experiments designed to differentiate between alterations in matter are fundamental to understanding basic chemistry. These experiments typically involve observing substances before and after undergoing processes like heating, cooling, mixing, or reacting with other substances. Changes in properties such as form, texture, color, odor, or the production of gas, light, or heat are carefully documented and analyzed to determine whether the underlying chemical composition has been altered or if the change is merely physical. For example, melting ice represents a physical change, as the water molecules remain H2O whether solid or liquid. Conversely, burning wood results in a chemical change, transforming the wood’s cellulose into ash, carbon dioxide, and water vapor.
Investigating the nature of these alterations provides a foundational understanding of how matter behaves under different conditions. Historically, the ability to distinguish between these transformations has been crucial for advancements in fields like materials science, medicine, and environmental science. This knowledge allows for the development of new materials with specific properties, the creation of life-saving pharmaceuticals, and a deeper understanding of environmental processes.
A thorough understanding of the distinction between these fundamental types of changes paves the way for more complex explorations of chemical reactions, stoichiometry, and the nature of chemical bonding. Further investigation often involves exploring specific types of reactions, such as combustion, precipitation, and acid-base reactions, as well as the energy changes associated with them.
Tips for Conducting Experiments on Material Transformations
Careful planning and execution are essential for successful laboratory investigations into the nature of changes in matter. Adhering to the following guidelines will ensure reliable results and a deeper understanding of the underlying chemical principles.
Tip 1: Precise Observation: Meticulous observation of initial and final states is crucial. Record all observable changes, including alterations in color, odor, texture, temperature, and the formation of precipitates or gases.
Tip 2: Controlled Experiments: Employ control groups whenever possible. A control group experiences all experimental conditions except the variable being tested, allowing for isolation of the variable’s impact.
Tip 3: Accurate Measurements: Use appropriate measuring instruments, such as graduated cylinders, balances, and thermometers, to ensure accurate quantitative data collection. Proper units should always be recorded.
Tip 4: Safety Precautions: Adhere to all laboratory safety protocols, including wearing appropriate personal protective equipment (PPE) like goggles and gloves. Consult safety data sheets (SDS) for all substances handled.
Tip 5: Systematic Documentation: Maintain a detailed laboratory notebook recording procedures, observations, data, and calculations. Clear and organized documentation is essential for accurate analysis and reproducibility.
Tip 6: Data Analysis: Analyze collected data to identify patterns and draw conclusions. Utilize tables, graphs, and calculations to interpret the results and determine the nature of the observed changes.
Careful attention to these guidelines will contribute to a deeper understanding of how matter interacts and transforms. These foundational concepts are essential for further studies in chemistry and related scientific disciplines.
By following these tips, one can effectively explore the fascinating world of chemical and physical transformations, laying the groundwork for future scientific inquiry.
1. Observation
Observation forms the cornerstone of scientific inquiry, particularly within the context of differentiating between physical and chemical changes in a laboratory setting. Accurate and detailed observation provides the empirical data necessary for drawing conclusions about the nature of transformations in matter.
- Qualitative Observations
Qualitative observations describe the qualities of a substance or process without numerical measurements. These include noting changes in color, odor, texture, and the formation of precipitates or gases. For example, observing a color change when two solutions are mixed suggests a possible chemical reaction, while a change in state from solid to liquid upon heating indicates a physical change.
- Quantitative Observations
Quantitative observations involve precise measurements using instruments. Recording the temperature change during a reaction, the mass of a substance before and after a process, or the volume of gas produced are examples of quantitative observations. These measurements provide crucial data for determining whether a change is physical or chemical. For instance, measuring the mass of a sample before and after melting confirms that mass is conserved in a physical change.
- Changes in State
Observing changes in the state of matter, such as melting, freezing, boiling, or condensation, is crucial for understanding physical changes. While the substance’s physical form alters, its chemical composition remains the same. Observing the transition of water from ice to liquid to vapor demonstrates this concept.
- Evidence of Chemical Reactions
Observations indicating a chemical reaction include the production of light or heat, formation of a precipitate, evolution of gas, or a permanent color change not associated with a change in state. For example, the burning of magnesium, producing bright light and heat, is a clear indicator of a chemical change.
The careful and systematic application of both qualitative and quantitative observation techniques in laboratory experiments allows for a nuanced understanding of the distinction between physical and chemical changes. By observing these transformations, scientists can gain valuable insights into the properties of matter and the nature of chemical reactions.
2. Experimentation
Experimentation is essential for distinguishing between physical and chemical changes in a laboratory setting. Well-designed experiments allow for controlled manipulation of variables and systematic observation of the resulting changes in matter, providing evidence to classify transformations as either physical or chemical.
- Controlled Variables
Manipulating one variable at a time while holding others constant is crucial. This isolates the impact of the specific variable on the substance. For example, in an experiment investigating the effect of temperature on a substance, only the temperature should be altered while other factors like pressure and volume remain constant. This controlled approach allows for clear determination of whether the observed change is due to the manipulated variable.
- Independent and Dependent Variables
Clearly identifying independent and dependent variables is essential. The independent variable is the one deliberately changed, while the dependent variable is the one measured or observed. In the example of heating a substance, temperature is the independent variable, and the observed change in state (solid, liquid, gas) is the dependent variable. Careful tracking of these variables allows for analysis of cause-and-effect relationships.
- Reproducibility
Experimental procedures must be documented precisely to ensure reproducibility. Detailed documentation enables other researchers to replicate the experiment and verify the results, strengthening the validity of the findings. This practice is fundamental to the scientific method and ensures that conclusions drawn about physical and chemical changes are reliable and robust.
- Data Collection and Analysis
Systematic data collection and analysis are critical for drawing valid conclusions. Quantitative data, such as temperature changes or mass measurements, should be recorded accurately using appropriate instruments. Qualitative data, like color changes or gas formation, should be described precisely. Analyzing this data allows for the identification of patterns and trends, ultimately determining whether the observed transformation indicates a physical or chemical change.
Through carefully controlled experimentation, employing precise measurements, and detailed data analysis, the distinction between physical and chemical changes can be clearly elucidated. This rigorous approach is fundamental to understanding the properties of matter and the nature of chemical reactions.
3. Matter Transformation
Matter transformation lies at the heart of distinguishing between physical and chemical changes within a laboratory setting. Investigating how matter changes form and composition provides crucial insights into the underlying nature of these transformations. Understanding these changes is fundamental to interpreting experimental results and drawing accurate conclusions about the properties of matter and the nature of chemical reactions.
- Physical Changes
Physical changes involve alterations in the form or state of matter without affecting its chemical composition. Examples include changes in state (melting, freezing, boiling, condensation), dissolving a solid in a liquid, and changes in shape or size. In a laboratory setting, observing these transformations, such as the melting of ice or the dissolving of salt in water, allows for the identification of characteristics of physical changes, like the reversibility of the process and the absence of new substance formation.
- Chemical Changes
Chemical changes, unlike physical changes, involve a change in the chemical composition of matter. This results in the formation of new substances with different properties. Common examples include combustion (burning), rusting, and cooking. Laboratory experiments focusing on chemical changes, such as the reaction of baking soda and vinegar or the burning of magnesium, demonstrate key characteristics, like the irreversibility of the process, the production of gas or light, and the formation of a precipitate.
- Conservation of Mass
The principle of conservation of mass states that matter cannot be created or destroyed in an isolated system, only transformed. This principle is crucial in laboratory investigations of both physical and chemical changes. In a physical change, the mass of the substance remains constant despite changes in form. In a chemical change, while the appearance of the substances involved may change, the total mass of the reactants equals the total mass of the products. This principle underscores the importance of precise measurements in laboratory experiments to verify the conservation of mass.
- Energy Changes
Both physical and chemical changes are accompanied by energy changes. Physical changes often involve relatively small energy changes, such as the heat absorbed during melting or released during freezing. Chemical changes, however, typically involve significant energy changes, either released (exothermic reactions, like combustion) or absorbed (endothermic reactions, like photosynthesis). Observing and measuring these energy changes in a laboratory context provide further evidence for classifying transformations as either physical or chemical. For instance, the temperature increase observed during an exothermic reaction clearly indicates a chemical change.
Analyzing matter transformations through the lens of physical and chemical changes, considering the conservation of mass and associated energy changes, allows for a deeper understanding of the fundamental principles governing the behavior of matter. This understanding is crucial for interpreting experimental results and making accurate conclusions in a “physical vs chemical change lab” setting. By carefully observing and analyzing these transformations, scientists can gain valuable insights into the properties of matter and the nature of chemical reactions.
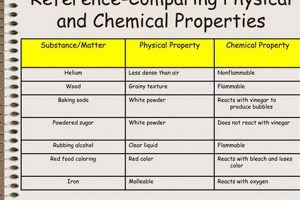
4. Property Analysis
Property analysis plays a crucial role in differentiating between physical and chemical changes in a laboratory setting. By examining specific properties of a substance before and after a transformation, one can gain valuable insights into the nature of the change. Alterations in physical properties, such as density, melting point, boiling point, and solubility, indicate a physical change, as the substance’s chemical composition remains constant. Conversely, changes in chemical properties, such as flammability, reactivity with acids or bases, and the formation of new substances, signify a chemical change, indicating an alteration in the substance’s chemical composition. For example, observing a change in the melting point of a substance after a process suggests a physical change, while observing the formation of a precipitate upon mixing two solutions indicates a chemical reaction.
The careful measurement and comparison of these properties are essential for accurate classification of the transformation. Precise measurement techniques, using instruments like thermometers, balances, and spectrometers, provide quantitative data that supports qualitative observations. For instance, measuring the density of a metal before and after heating can confirm whether a physical change has occurred, while observing a change in the pH of a solution after adding an acid signifies a chemical change. Understanding the specific properties affected and the extent of these changes allows for a more nuanced understanding of the transformation. This analytical approach is crucial for interpreting experimental results and drawing valid conclusions.
In conclusion, property analysis provides a powerful tool for distinguishing between physical and chemical changes in a laboratory environment. The systematic examination of both physical and chemical properties before and after a transformation offers essential evidence for classifying the nature of the change. This understanding is not only crucial for basic scientific inquiry but also has practical implications in various fields, including materials science, environmental monitoring, and chemical engineering. Challenges in property analysis can arise from the complexity of some substances and the limitations of measurement techniques, but advancements in analytical methods continue to refine our ability to understand the intricate nature of matter transformations.
5. Reaction Identification
Reaction identification is integral to the “physical vs chemical change lab” experience. Accurately classifying observed transformations necessitates discerning the specific type of reaction occurring, if any. This identification relies on observing characteristic indicators. For instance, gas evolution suggests potential decomposition or single/double displacement reactions. Precipitate formation points towards double displacement reactions. Color changes may signal redox reactions or complexation. Exothermic or endothermic observations suggest energy transfer typical of many chemical changes. Correctly categorizing these observations as indicative of specific reaction types strengthens the analysis, moving beyond merely labeling a change as “chemical” and towards a more nuanced understanding of the underlying chemical processes. For example, observing the effervescence of carbon dioxide when hydrochloric acid is added to calcium carbonate clearly indicates a chemical change driven by a double displacement reaction. Conversely, dissolving sugar in water, despite appearing visually similar to the acid-base reaction, lacks the same chemical indicators and is correctly identified as a physical change.
This ability to identify specific reaction types enhances experimental interpretation. Recognizing a reaction as a combustion process, for instance, informs expectations regarding the products and energy changes involved. Similarly, identifying a precipitation reaction allows for predictions about the solubility of the products and potential stoichiometric calculations. These insights are fundamental to advancing from a basic understanding of change to a more sophisticated comprehension of chemical reactivity. This knowledge has practical applications in fields like materials science, where understanding reaction mechanisms is crucial for designing new materials with specific properties. In environmental science, reaction identification aids in understanding pollution formation and mitigation strategies.
In summary, reaction identification elevates the “physical vs chemical change lab” from simple observation to informed analysis. Connecting observed changes to specific reaction types provides a deeper understanding of the underlying chemical principles. This sophisticated approach to laboratory investigation fosters stronger analytical skills applicable to a wide range of scientific disciplines. However, challenges remain in accurately identifying reactions in complex systems or when multiple reactions occur simultaneously. Ongoing development of analytical techniques and a deeper understanding of chemical principles continually refine the ability to effectively identify and interpret reactions in diverse laboratory settings.
6. Safety Procedures
Safety procedures are paramount in any laboratory setting, especially when investigating physical and chemical changes. Experiments often involve potentially hazardous substances and procedures. Therefore, adherence to established safety protocols is not merely a recommendation, but a critical prerequisite for responsible scientific practice. Direct handling of chemicals necessitates appropriate personal protective equipment (PPE), including gloves and eye protection. Furthermore, understanding the potential hazards associated with specific chemicals, such as flammability, corrosiveness, or toxicity, is essential. Consulting Safety Data Sheets (SDS) before handling any chemical provides crucial information regarding safe handling, storage, and emergency procedures. For instance, experiments involving flammable liquids require specific precautions to prevent ignition sources and appropriate fire suppression equipment nearby. Ignoring such precautions could lead to serious accidents, highlighting the direct link between safety procedures and the integrity of the “physical vs chemical change lab” environment.
The importance of safety extends beyond personal protection to encompass the broader laboratory environment. Proper waste disposal procedures are crucial for preventing environmental contamination and ensuring the safety of others. Specific waste streams must be designated for different types of chemicals, such as heavy metals or organic solvents. Neutralizing reactive substances before disposal is often necessary to minimize hazards. For example, strong acids should be neutralized with a base before disposal. Furthermore, maintaining a clean and organized workspace is essential for preventing accidents and ensuring accurate experimental results. Cluttered workspaces can lead to spills, cross-contamination, and difficulty in locating necessary equipment during an emergency. Therefore, a well-maintained laboratory environment is not merely aesthetically pleasing but a critical component of a comprehensive safety strategy.
In conclusion, safety procedures form an inseparable component of the “physical vs chemical change lab.” Neglecting these procedures jeopardizes not only personal safety but also the validity of experimental results and the overall integrity of the scientific process. Understanding the specific hazards associated with each experiment and adhering to appropriate safety protocols is not merely a matter of compliance, but a demonstration of responsible scientific practice. While established safety procedures provide a strong foundation, ongoing vigilance and adaptation to specific experimental conditions are essential. Challenges remain in ensuring consistent adherence to safety protocols and adapting to evolving safety standards. However, a culture of prioritizing safety, alongside rigorous training and ongoing evaluation of safety practices, minimizes risks and fosters a productive and secure laboratory environment.
Frequently Asked Questions
Addressing common inquiries regarding the differentiation between physical and chemical changes in a laboratory setting is crucial for fostering a comprehensive understanding of these fundamental concepts.
Question 1: How can one definitively determine if a change is physical or chemical?
The definitive test lies in whether new substances are formed. A physical change alters the form or state of matter without affecting its chemical composition. A chemical change, however, results in the formation of new substances with different properties.
Question 2: Is dissolving a substance in water a physical or chemical change?
Dissolving a substance like sugar in water is generally a physical change. While the sugar becomes dispersed within the water, its chemical composition remains unchanged. It can be recovered by evaporating the water.
Question 3: Are all chemical changes irreversible?
Most chemical changes are practically irreversible. While some reactions can theoretically be reversed, the conditions required are often not easily attainable in typical laboratory settings.
Question 4: How does observing energy changes aid in classifying a transformation?
Significant energy changes, typically in the form of heat or light emission or absorption, often accompany chemical changes. While physical changes may involve energy transfer, the magnitude is usually considerably smaller.
Question 5: Why is precise measurement important in distinguishing these changes?
Precise measurement is essential for verifying the conservation of mass, a fundamental principle in both physical and chemical changes. Accurate mass measurements before and after a transformation help confirm whether new substances have been formed.
Question 6: Can a physical change lead to a chemical change?
While a physical change itself does not alter chemical composition, it can create conditions that facilitate a subsequent chemical change. For example, increasing the surface area of a substance by grinding it can increase its reactivity and make it more susceptible to chemical change.
Understanding these key distinctions is crucial for interpreting experimental results and drawing valid conclusions about matter transformations. This knowledge forms the basis for further exploration into the complexities of chemical reactions and their applications in various scientific fields.
Further investigation often involves exploring specific types of reactions and the underlying chemical principles governing them. This may include topics such as stoichiometry, reaction kinetics, and thermodynamics.
Conclusion
Laboratory exploration of physical versus chemical changes provides a fundamental framework for understanding matter transformations. Careful observation, meticulous experimentation, and precise property analysis are crucial for distinguishing between alterations in form and alterations in composition. Identification of specific reaction types deepens this understanding, allowing for more nuanced interpretations of observed phenomena. Stringent adherence to safety procedures ensures the integrity of experimental results and safeguards the laboratory environment. This foundational knowledge serves as a cornerstone for more advanced studies in chemistry and related scientific disciplines.
The ability to differentiate between these fundamental changes is not merely an academic exercise; it has profound implications across diverse scientific fields. From designing new materials to understanding environmental processes, this knowledge empowers scientific advancements and fosters a deeper appreciation for the intricate nature of the material world. Continued investigation into the complexities of chemical and physical transformations promises to unlock further insights into the fundamental principles governing the universe and drive innovation across various disciplines. Rigorous laboratory practices and a commitment to scientific inquiry remain essential for unlocking the full potential of this knowledge and addressing future scientific challenges.