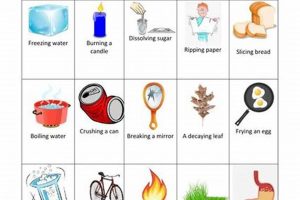
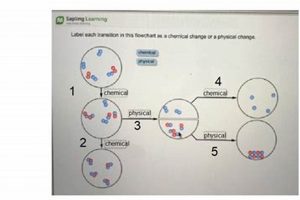
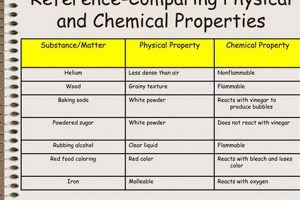
Experiments designed to differentiate between alterations in matter that affect only physical properties, such as shape or state, and those that result in new substances with different chemical compositions are foundational in scientific education. These investigations typically involve observing substances before, during, and after subjecting them to various procedures like heating, cooling, mixing, or dissolving. For instance, melting ice illustrates a shift in state without altering the underlying water molecules, while burning wood produces ash and gases, signifying a change in chemical composition.
Such experimentation provides a crucial understanding of the nature of matter and its interactions. It fosters critical thinking skills by requiring students to analyze observations and draw conclusions about the type of change observed. Historically, the ability to distinguish between these two fundamental processes has been essential for advancements in fields ranging from metallurgy to medicine, laying the groundwork for our modern understanding of chemical reactions and material science.
This foundation in differentiating these changes forms the basis for exploring more advanced concepts in chemistry, physics, and other scientific disciplines. Topics such as reaction rates, energy transfer, and the properties of different states of matter all build upon this fundamental understanding of how matter behaves under various conditions.
Tips for Effective Experimentation
Careful planning and execution are essential for successful experimentation to determine changes in matter. The following tips facilitate accurate observations and conclusions.
Tip 1: Precise Observation: Meticulous documentation of initial appearances, including color, texture, and state, is crucial for comparison with post-experiment observations.
Tip 2: Controlled Experiments: Isolating the variable under investigation ensures that observed changes can be attributed to the specific manipulation. Control groups provide a baseline for comparison.
Tip 3: Appropriate Safety Measures: Experiments involving heat, chemicals, or glassware require appropriate safety precautions, including eye protection and proper ventilation.
Tip 4: Accurate Measurements: Quantitative data, such as mass and temperature changes, provide valuable evidence for identifying the type of change. Calibrated instruments are essential for accurate measurements.
Tip 5: Systematic Data Recording: Organized recording of observations and measurements in a dedicated lab notebook facilitates analysis and interpretation.
Tip 6: Consideration of Reversibility: Exploring whether a change can be reversed back to its original state offers a key insight into its nature. Physical changes are often easily reversed, while chemical changes typically are not.
Tip 7: Disposal of Materials: Proper disposal of materials according to safety guidelines is crucial for maintaining a safe laboratory environment.
Adhering to these guidelines enhances experimental accuracy and contributes to a deeper understanding of the nature of matter and its transformations.
By incorporating these principles, investigations into changes in matter can provide valuable insights into fundamental scientific concepts.
1. Observation
Observation forms the cornerstone of any investigation into the nature of changes in matter. Within the context of differentiating between physical and chemical changes, careful observation provides the empirical data upon which conclusions are drawn. Changes in properties such as color, odor, temperature, the formation of a precipitate, or the evolution of a gas serve as crucial indicators of transformation. For example, the observation that iron rusts, accompanied by a color change and the formation of a flaky substance, suggests a chemical change. Conversely, observing that ice melts, transitioning from solid to liquid without altering its fundamental composition, signifies a physical change. The accuracy and detail of observations directly impact the validity of inferences regarding the type of change that has occurred.
The importance of observation extends beyond merely noting changes. Quantitative observations, such as measuring temperature variations during a reaction or determining the mass of substances before and after an interaction, provide quantifiable evidence for analysis. Furthermore, observing the reversibility of a process plays a critical role. For instance, if a substance returns to its original state after a change, as observed when water condenses back into liquid form after boiling, this supports the classification of a physical change. Conversely, the inability to reverse a process, such as the burning of wood, further strengthens the identification of a chemical change. These observational nuances provide essential data points for accurate categorization.
Developing keen observational skills is paramount for accurate scientific inquiry. Thorough and systematic documentation of all observed changes, both qualitative and quantitative, ensures reliable and reproducible results. This meticulous approach to observation strengthens the validity of experimental conclusions and allows for a deeper understanding of the fundamental principles governing the behavior of matter during physical and chemical transformations. Challenges in observation can arise from subtle changes or limitations in detection methods, emphasizing the need for both careful experimental design and the appropriate use of instrumentation. Overcoming these challenges reinforces the crucial role observation plays in advancing scientific knowledge.
2. Experimentation
Experimentation serves as the cornerstone of investigations into physical and chemical changes, providing a structured framework for manipulating variables and observing outcomes. Within the context of a physical or chemical change lab, experimentation enables researchers to test hypotheses about the nature of transformations. Manipulating variables, such as temperature or the introduction of another substance, allows for the controlled observation of changes in the properties of matter. For instance, heating a sample of ice allows for observation of its transition from solid to liquid, showcasing a physical change. Conversely, burning magnesium in the presence of oxygen produces a bright light and a white powder (magnesium oxide), indicating a chemical change. These controlled manipulations provide direct evidence for the classification of changes.
The design of experiments in this domain often involves comparing the behavior of a control group with an experimental group, ensuring that observed changes can be directly attributed to the manipulated variable. For example, comparing the mass of a sample of sugar before and after dissolving it in water allows for the determination of whether a chemical change has occurred. If the mass remains consistent after evaporation of the water, this supports the classification as a physical change. Experimentation also allows for the exploration of reversibility. Melting ice can be reversed by cooling, indicating a physical change. However, the combustion of wood is irreversible, characteristic of a chemical change. These experimental approaches offer valuable insights into the fundamental differences between these two types of transformations.
Effective experimentation relies on meticulous planning, precise measurements, and accurate recording of observations. Challenges may arise from controlling all variables or accurately measuring changes. However, careful experimental design and execution minimize these limitations, leading to robust conclusions. Understanding the role of experimentation in the investigation of physical and chemical changes provides a foundation for more complex scientific inquiries and allows for a deeper understanding of the properties of matter and their interactions.
3. Analysis
Analysis forms an integral component of investigations into physical and chemical changes, providing the framework for interpreting experimental observations and drawing meaningful conclusions. Within the context of a physical or chemical change lab, analysis involves scrutinizing collected data, including qualitative observations and quantitative measurements, to determine the nature of the transformations that have occurred. For example, observing the formation of a precipitate upon mixing two solutions, coupled with an analysis of the chemical composition of the precipitate, strongly suggests a chemical change. Conversely, observing a change in state, such as the melting of ice, coupled with an analysis demonstrating that the chemical composition remains constant, indicates a physical change. This analytical process bridges the gap between observation and understanding.
The importance of analysis lies in its ability to distinguish between superficial changes and those that alter the fundamental composition of matter. Analyzing the mass of reactants and products in a chemical reaction allows for the determination of whether mass is conserved, a key principle in chemistry. Similarly, analyzing the energy changes associated with a physical change, such as the heat absorbed during melting or released during freezing, provides insights into the thermodynamic properties of substances. Analysis may involve calculations, graphing, or comparing experimental results to established scientific principles. For instance, analyzing the pH of a solution before and after a reaction can indicate whether a chemical change has taken place. These analytical tools empower researchers to move beyond simple observations and gain a deeper understanding of the underlying processes governing transformations.
Challenges in analysis can arise from the complexity of the system under study, limitations in measurement accuracy, or the presence of confounding variables. However, careful experimental design and rigorous data analysis mitigate these challenges, enhancing the validity of conclusions. The practical significance of this analytical approach extends beyond the laboratory setting, informing advancements in fields such as materials science, environmental science, and medicine. A strong understanding of the principles of analysis empowers researchers to interpret experimental findings critically and contribute to a more profound understanding of the nature of physical and chemical changes in the world around us.
4. Classification
Classification represents a crucial step in any investigation involving physical and chemical changes, providing a framework for organizing observations and experimental results into meaningful categories. Within the context of a physical or chemical change lab, classification involves assigning observed transformations to one of two fundamental categories: physical changes, which alter the form or state of matter without affecting its chemical composition, and chemical changes, which result in the formation of new substances with different properties. This categorization relies on a careful analysis of experimental data, including observations of changes in properties such as color, odor, temperature, and the formation of precipitates or gases. For example, the melting of ice, which involves a change in state from solid to liquid without altering the chemical composition of water, is classified as a physical change. Conversely, the burning of wood, which produces ash, smoke, and heat, and results in the formation of new substances, is classified as a chemical change. Accurate classification is essential for understanding the underlying processes governing these transformations.
The importance of classification stems from its ability to provide a structured approach to understanding the diverse ways in which matter can interact and transform. Classifying a change as physical or chemical allows for predictions about its behavior and properties. For instance, physical changes are often easily reversible, as demonstrated by the freezing and melting of water. Chemical changes, on the other hand, are typically not easily reversed, as exemplified by the combustion of gasoline. This predictive power is invaluable in various scientific disciplines, including materials science, where understanding the type of change occurring during material processing is crucial for controlling the final product’s properties. Furthermore, proper classification allows for the application of appropriate scientific principles. Physical changes are governed by principles of thermodynamics, while chemical changes are governed by principles of chemical kinetics and stoichiometry. This distinction is crucial for accurate interpretation and analysis of experimental data.
Challenges in classification can arise from complex processes involving both physical and chemical changes occurring simultaneously or from limitations in experimental techniques. However, careful experimental design, rigorous data analysis, and a thorough understanding of the underlying scientific principles allow for accurate classification in most cases. The ability to classify changes accurately underpins advancements in diverse fields, ranging from the development of new materials to the understanding of biological processes. A strong grasp of the principles of classification empowers researchers to interpret experimental findings effectively and contribute to a more comprehensive understanding of the nature of matter and its transformations.
5. Matter Interaction
Matter interaction lies at the heart of understanding physical and chemical changes, providing the fundamental basis for observing and interpreting transformations in a laboratory setting. Investigating how matter interacts with itself and its surroundings is crucial for distinguishing between changes that alter only physical properties and those that result in new substances with different chemical compositions. This exploration delves into the various facets of matter interaction, highlighting their relevance to differentiating between these two fundamental types of changes.
- Intermolecular Forces
Intermolecular forces play a significant role in physical changes. These forces, which exist between molecules, influence properties such as melting point and boiling point. For example, the relatively weak intermolecular forces in ice allow it to melt into liquid water at a relatively low temperature. In a physical or chemical change lab, observing changes in state, driven by alterations in intermolecular forces, provides crucial evidence for classifying transformations as physical changes. The strength of these forces determines the energy required to induce phase transitions.
- Chemical Bonding
Chemical bonding, the interaction of atoms through the sharing or transfer of electrons, is central to chemical changes. The breaking and formation of chemical bonds result in new substances with different properties. The combustion of methane, for example, involves the breaking of carbon-hydrogen and oxygen-oxygen bonds and the formation of carbon-oxygen and hydrogen-oxygen bonds, producing carbon dioxide and water. In a laboratory setting, evidence of new bond formation, such as the evolution of gas or the formation of a precipitate, serves as a key indicator of a chemical change.
- Collision Theory
Collision theory explains how chemical reactions occur at the molecular level. It postulates that reactions proceed through the collision of reactant molecules with sufficient energy and proper orientation. Factors influencing reaction rates, such as temperature and concentration, can be understood through the lens of collision theory. In a laboratory setting, manipulating these factors allows for controlled experimentation and observation of reaction rates, furthering the understanding of chemical changes.
- Energy Transfer
Energy transfer is a critical component of both physical and chemical changes. Physical changes often involve the absorption or release of heat, as seen in the melting of ice or the condensation of water vapor. Chemical changes, on the other hand, can involve significant energy changes, either releasing energy (exothermic reactions) or absorbing energy (endothermic reactions). Observing and quantifying energy changes during experiments provide valuable insights into the nature of the transformations occurring within a physical or chemical change lab.
Understanding these facets of matter interaction provides a fundamental basis for interpreting observations and drawing conclusions in a physical or chemical change lab. By analyzing experimental data through the lens of intermolecular forces, chemical bonding, collision theory, and energy transfer, researchers can confidently distinguish between physical and chemical changes, furthering their understanding of the properties of matter and the transformations it undergoes.
6. Safety Procedures
Safety procedures are paramount in environments involving investigations into the nature of matter, such as a physical or chemical change lab. These procedures mitigate risks inherent in handling chemicals and equipment, ensuring the well-being of all individuals involved. A comprehensive understanding of potential hazards and the implementation of appropriate safety measures are crucial for preventing accidents and promoting a secure laboratory environment. Ignoring safety protocols can lead to significant consequences, ranging from minor injuries to severe accidents. For instance, handling corrosive chemicals without proper gloves can cause burns, while neglecting proper ventilation can lead to inhalation of toxic fumes. The cause-and-effect relationship between adherence to safety protocols and a secure working environment is undeniable.
Safety procedures function as an integral component of a physical or chemical change lab, directly impacting the reliability and validity of experimental results. A safe laboratory environment ensures that experiments are conducted under controlled conditions, minimizing the risk of extraneous factors influencing outcomes. This control contributes to accurate data collection and meaningful interpretation of results. Furthermore, adherence to safety procedures instills a culture of responsibility and promotes careful experimental design, minimizing errors and maximizing the value of scientific inquiry. Real-life examples underscore the practical significance of this understanding. Proper disposal of chemical waste prevents environmental contamination, while the use of safety goggles protects against eye injuries from chemical splashes or shattered glassware. These practical applications highlight the essential role of safety procedures in maintaining both personal safety and the integrity of scientific investigations.
In conclusion, safety procedures form an indispensable aspect of any laboratory environment investigating physical and chemical changes. Their meticulous implementation fosters a secure working environment, safeguarding individuals and promoting reliable experimental outcomes. Challenges in maintaining consistent adherence to safety protocols necessitate ongoing training, regular inspections, and a proactive approach to risk management. Addressing these challenges ensures that laboratories remain safe spaces for scientific exploration and discovery. The overarching goal is to create an environment where scientific inquiry can flourish without compromising the well-being of individuals or the integrity of the research itself.
7. Scientific Method
The scientific method provides the essential framework for conducting rigorous and reproducible experiments within a physical or chemical change lab. Its structured approach ensures systematic observation, hypothesis formulation, experimental design, data analysis, and conclusion drawing. Employing the scientific method strengthens the validity of experimental results and fosters a deeper understanding of the underlying principles governing matter transformations. For instance, when investigating whether heating sugar results in a physical or chemical change, the scientific method guides researchers through a series of steps: initial observation of the sugar’s properties, formulation of a hypothesis about the effect of heat, designing a controlled experiment to heat the sugar while monitoring changes, meticulous data collection (e.g., color change, odor, mass), analysis of the data to determine if a new substance was formed, and finally, drawing conclusions based on the evidence. Adhering to this structured approach allows for robust and trustworthy results. This cause-and-effect relationship between utilizing the scientific method and achieving reliable experimental outcomes is fundamental to scientific inquiry.
The scientific method’s importance as a component of investigations into physical and chemical changes lies in its ability to minimize bias and maximize objectivity. By requiring a systematic approach to experimentation, the scientific method reduces the influence of preconceived notions or subjective interpretations, leading to more accurate conclusions. For instance, if a researcher believes that mixing two clear liquids will always result in a physical change, the scientific method compels them to conduct the experiment objectively, carefully observing and recording any changes in properties, such as the formation of a precipitate or a change in temperature, which might indicate a chemical change instead. Real-life examples abound. Consider the historical debate about the nature of combustion. Through meticulous experimentation guided by the scientific method, scientists disproved the phlogiston theory and established the role of oxygen in combustion. This historical example illustrates the power of the scientific method in overturning established but incorrect theories and advancing scientific understanding.
A deep understanding of the scientific method and its application in the context of physical and chemical change labs allows for rigorous investigation of matter’s properties and transformations. Challenges can arise in applying the scientific method perfectly, such as limitations in experimental design or the presence of confounding variables. However, recognizing these challenges and striving for meticulous execution of the scientific method strengthens the validity of research findings and contributes to a more comprehensive understanding of the physical and chemical world. This understanding is fundamental not only to scientific advancement but also to technological innovation and informed decision-making in various fields, from materials science to environmental science.
Frequently Asked Questions
This section addresses common inquiries regarding the differentiation between physical and chemical changes, providing concise and informative responses to enhance understanding of these fundamental concepts.
Question 1: How can one definitively determine if a change is physical or chemical?
The definitive test lies in whether new substances are formed. A chemical change results in new substances with different chemical compositions and properties, while a physical change alters only the form or state of a substance without affecting its composition. Chemical changes often involve observable phenomena like gas production, precipitate formation, or significant color change, whereas physical changes usually manifest as alterations in shape, size, or state of matter.
Question 2: Is dissolving sugar in water a chemical or physical change?
Dissolving sugar in water is a physical change. While the sugar becomes dispersed within the water, its chemical composition remains unchanged. The sugar molecules are merely separated and surrounded by water molecules; they do not react to form new substances. This can be confirmed by evaporating the water, which leaves the sugar behind in its original form.
Question 3: Why is burning wood considered a chemical change?
Burning wood is a chemical change because it results in the formation of new substances. The wood reacts with oxygen in the air to produce ash, smoke (containing various gases like carbon dioxide and water vapor), and heat. The original wood is transformed into different substances with distinct chemical compositions.
Question 4: Can a change exhibit characteristics of both physical and chemical changes?
Certain processes may exhibit characteristics of both. For example, dissolving a metal in acid involves a physical change (the metal dissolving) and a chemical change (the metal reacting with the acid to produce a salt). Distinguishing the dominant change requires careful analysis of the overall transformation.
Question 5: What role does energy play in differentiating between these changes?
Energy changes accompany both physical and chemical changes. However, chemical changes typically involve more significant energy changes, often observed as heat released (exothermic reactions) or absorbed (endothermic reactions). Physical changes generally involve smaller energy changes associated with changes in state, such as the heat absorbed during melting or released during freezing.
Question 6: How are these concepts applied in real-world scenarios?
Understanding physical and chemical changes is crucial in numerous fields. Cooking relies on chemical changes to transform ingredients, while material science utilizes knowledge of these changes to develop new materials with specific properties. Environmental science applies these concepts to understand processes like the rusting of iron or the decomposition of organic matter. Medicine relies on understanding chemical changes in the body for drug development and diagnostics.
A thorough understanding of these concepts provides a foundation for further exploration of the properties of matter and their interactions.
This FAQ section has provided answers to common queries regarding physical and chemical changes. Further exploration of specific examples and experimental techniques can deepen understanding and facilitate more advanced study.
Conclusion
Investigations into differentiating between physical and chemical changes provide a foundational understanding of matter’s behavior. Careful observation, meticulous experimentation, and rigorous analysis empower differentiation between transformations affecting only physical properties and those resulting in new substances. From the melting of ice to the burning of magnesium, exploring these changes illuminates fundamental principles governing the interactions and transformations of matter. A thorough grasp of these concepts equips individuals with the ability to interpret observations, predict outcomes, and appreciate the dynamic nature of the material world.
Continued exploration of physical and chemical changes remains crucial for advancements across scientific disciplines. From developing novel materials to understanding complex biological processes, this fundamental knowledge underpins progress in diverse fields. Further investigation into the intricacies of matter interaction, coupled with technological advancements in analytical techniques, promises to deepen comprehension of these transformative processes and unlock new possibilities in scientific discovery and innovation.