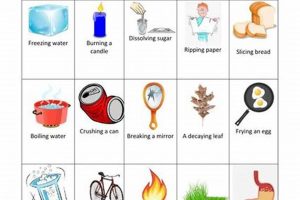
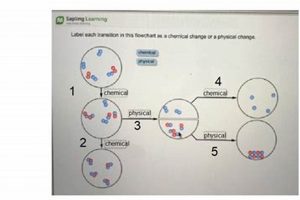
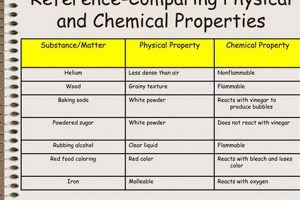
Laboratory investigations into transformations of matter provide foundational knowledge in the field of chemistry. These transformations are broadly categorized as either physical, involving alterations in form or state without changing the substance’s chemical identity (e.g., melting ice), or chemical, involving the formation of new substances with different properties (e.g., burning wood). Practical experimentation allows for observation and measurement of characteristics like temperature, pressure, and volume changes, providing empirical evidence to support theoretical understanding. Examples include observing phase transitions, identifying reaction products, and measuring energy changes associated with reactions.
Understanding these fundamental processes is critical for a wide range of scientific disciplines, including materials science, medicine, and environmental science. Historically, systematic study of such transformations has led to advancements in areas like pharmaceuticals, polymer development, and energy production. By elucidating the principles governing these changes, researchers can design new materials with specific properties, develop more efficient chemical processes, and gain deeper insights into the natural world.
This exploration will delve further into specific experimental techniques, common laboratory procedures for observing these transformations, and the theoretical principles underlying these observations. It will also highlight the connections between laboratory findings and real-world applications.
Tips for Investigating Transformations of Matter in the Laboratory
Careful laboratory practices are essential for obtaining accurate results and ensuring safety when investigating changes in matter. The following tips provide guidance for effective experimentation.
Tip 1: Precise Observation: Detailed record-keeping is crucial. Note initial states, any changes observed during the process (color change, gas evolution, temperature fluctuations), and final states. Use appropriate instruments for quantitative measurements.
Tip 2: Control Experiments: Utilize control experiments to isolate variables and establish cause-and-effect relationships. A control experiment should replicate all conditions except the one being tested.
Tip 3: Proper Handling of Chemicals: Adhere to safety guidelines and use appropriate personal protective equipment (PPE). Consult safety data sheets (SDS) before handling any chemicals. Dispose of waste materials according to established procedures.
Tip 4: Accurate Measurements: Utilize appropriate measuring instruments (graduated cylinders, balances, thermometers) and ensure they are properly calibrated. Record measurements with the correct number of significant figures.
Tip 5: Experimental Design: Develop a clear experimental plan before commencing work. Outline the procedures, identify necessary materials, and consider potential sources of error.
Tip 6: Data Analysis: Analyze collected data to identify trends and draw conclusions. Use appropriate graphical representations and statistical methods when applicable. Compare experimental findings with theoretical predictions.
Tip 7: Cleanliness and Organization: Maintaining a clean and organized workspace promotes both safety and accuracy. Ensure all equipment is clean and properly stored after use.
Adherence to these practices ensures the reliability of experimental results and contributes to a safer laboratory environment. Accurate data collection and analysis are essential for furthering scientific understanding of the fundamental principles governing transformations of matter.
These laboratory techniques and safety protocols are fundamental to conducting meaningful scientific investigations into the nature of chemical and physical changes. The next section will discuss specific examples of laboratory experiments and their applications.
1. Observation
Observation forms the cornerstone of scientific inquiry, particularly in the study of physical and chemical changes within a laboratory setting. Accurate and detailed observation provides the raw data upon which analysis, interpretation, and ultimately, understanding are built. It bridges the gap between theoretical concepts and experimental reality, enabling researchers to discern patterns, identify anomalies, and draw meaningful conclusions about the nature of matter and its transformations.
- Qualitative Observations
Qualitative observations describe the qualities of a substance or process without numerical measurements. These include noting color changes (e.g., a solution turning blue), changes in state (e.g., a solid melting into a liquid), the formation of a precipitate (e.g., a cloudy suspension forming in a clear solution), or the evolution of a gas (e.g., bubbles forming during a reaction). In the context of studying transformations, qualitative observations provide initial evidence of change and can guide further, more quantitative investigation.
- Quantitative Observations
Quantitative observations involve numerical measurements using instruments. Examples include measuring temperature changes with a thermometer (e.g., a temperature increase during an exothermic reaction), mass changes with a balance (e.g., mass loss during the decomposition of a compound), volume changes with a graduated cylinder (e.g., volume increase of a gas produced), or pH changes with a pH meter. These precise measurements allow for a more rigorous analysis of the changes occurring and provide data that can be used to support or refute hypotheses.
- Systematic Observation
Systematic observation involves following a structured approach to ensure comprehensive data collection. This might involve using a checklist, following a specific experimental protocol, or recording observations at predetermined intervals. In the context of transformations, systematic observation ensures that all relevant data points are captured, minimizing the risk of overlooking subtle but important changes and promoting reproducibility of the experiment.
- Objective Observation
Objective observation requires separating personal biases and interpretations from the recorded data. This involves focusing solely on factual details and avoiding subjective descriptions or inferences. For example, instead of stating “the reaction occurred quickly,” an objective observer would note “the reaction completed within 10 seconds.” This objectivity is crucial for ensuring the integrity of scientific investigations and allowing for unbiased interpretation of results.
The integration of these different facets of observation within a controlled laboratory environment is crucial for gaining a comprehensive understanding of physical and chemical changes. The ability to accurately observe, record, and analyze these changes allows researchers to develop and refine models of how matter behaves, leading to advancements in various fields, from materials science to medicine.
2. Measurement
Measurement plays a crucial role in the investigation of physical and chemical changes within a laboratory setting. Quantitative data acquired through precise measurement provides the empirical evidence necessary to understand and interpret these transformations. This data allows for the identification of patterns, the establishment of relationships between variables, and the development of predictive models. For example, measuring the temperature change during a chemical reaction provides insights into the energy transfer associated with the reaction, distinguishing exothermic from endothermic processes. Similarly, measuring the mass of reactants and products allows for the determination of stoichiometric relationships and assessment of reaction efficiency. Without accurate measurement, observations remain qualitative and lack the precision necessary for deeper scientific understanding.
Different types of measurements are relevant depending on the specific transformation under investigation. Changes in mass, volume, temperature, pressure, pH, and conductivity can all provide valuable information about the nature of a physical or chemical change. Utilizing appropriate instruments and techniques is crucial for obtaining reliable and meaningful data. For instance, a calibrated balance is essential for accurate mass measurements, while a calibrated thermometer is required for precise temperature readings. Furthermore, understanding the limitations of each instrument and the potential sources of error is critical for proper data interpretation. In the case of volume measurements, using the appropriate glassware, such as a graduated cylinder or burette, and accounting for meniscus effects, ensures accuracy. When measuring pH changes, proper calibration and maintenance of the pH meter are essential for obtaining reliable results.
The practical significance of accurate measurement in the study of transformations extends beyond the laboratory. In industrial settings, precise measurements are essential for process control, quality assurance, and optimization of chemical reactions. For example, in the pharmaceutical industry, precise measurements are critical for ensuring drug purity and efficacy. In environmental monitoring, accurate measurements of pollutants are essential for assessing environmental impact and developing remediation strategies. The ability to accurately measure physical and chemical changes is thus fundamental to advancements in various scientific and technological fields, contributing to both theoretical understanding and practical applications.
3. Experimentation
Experimentation forms the core of scientific investigation into physical and chemical changes, providing a structured framework for testing hypotheses, gathering empirical data, and refining theoretical understanding. It bridges the gap between observation and interpretation, allowing researchers to manipulate variables, observe outcomes, and establish causal relationships. In the context of laboratory chemistry, well-designed experiments allow for the controlled investigation of transformations, enabling isolation of specific variables and precise measurement of effects.
A classic example is the investigation of reaction rates. By systematically varying reactant concentrations, temperature, or the presence of catalysts, while meticulously monitoring reaction progress, researchers can determine the factors influencing reaction speed and develop rate laws. Such experimental control is essential for distinguishing between correlation and causation, ensuring that observed changes are directly attributable to manipulated variables. Similarly, experiments investigating electrolysis, where an electric current drives a chemical change, illustrate the power of controlled experimentation in elucidating the nature of chemical transformations and their underlying mechanisms.
Furthermore, experimentation provides insights into the practical implications of chemical and physical changes. Studies on material properties, such as tensile strength or thermal conductivity, rely heavily on controlled experiments to understand how these properties change under different conditions. This understanding is crucial for material selection and design in engineering applications. Similarly, the development of new pharmaceuticals involves rigorous experimentation to assess drug efficacy and safety, demonstrating the crucial role of experimentation in advancing scientific knowledge and its practical applications.
Careful experimental design, execution, and data analysis are essential for drawing valid conclusions. Controlling for extraneous variables, ensuring reproducibility, and utilizing appropriate statistical methods are crucial for the reliability of experimental results. Moreover, ethical considerations, particularly when dealing with potentially hazardous materials or biological systems, are paramount in scientific experimentation. By adhering to rigorous standards, experimental investigations provide the robust evidence necessary to advance our understanding of chemical and physical changes and their impact on the world around us.
4. Analysis
Analysis forms an integral part of investigating physical and chemical changes within a laboratory setting. It’s the bridge connecting raw experimental data to meaningful scientific conclusions. Analysis provides the tools and methodologies to interpret observations, identify patterns, and extract valuable insights from experimental results. Without rigorous analysis, data remains merely a collection of numbers and observations, devoid of scientific significance. In the context of transformations of matter, analysis clarifies the nature of the changes observed, quantifies the extent of these changes, and establishes relationships between different variables.
- Data Interpretation
Data interpretation involves transforming raw data into meaningful information. This includes using graphical representations, statistical methods, and chemical calculations to identify trends, patterns, and anomalies. For example, plotting reaction rate data against reactant concentration can reveal the order of a reaction, while statistical analysis can determine the significance of observed differences between experimental groups. In studies of phase changes, analyzing temperature-time data reveals distinct plateaus corresponding to melting and boiling points.
- Stoichiometric Calculations
Stoichiometry provides the quantitative framework for analyzing chemical reactions. Calculating theoretical yields, percent yields, and limiting reactants enables assessment of reaction efficiency and understanding of reactant proportions. In combustion analysis experiments, stoichiometry allows for the determination of the empirical formula of an unknown compound based on the masses of its combustion products. Similarly, in titrations, stoichiometric calculations determine the concentration of an analyte based on the volume and concentration of the titrant.
- Error Analysis
Error analysis is crucial for assessing the reliability and validity of experimental results. Identifying potential sources of error, both systematic and random, and quantifying their impact allows researchers to determine the uncertainty associated with measurements and calculations. Understanding error margins strengthens the interpretation of experimental outcomes. For instance, in calorimetry experiments, accounting for heat loss to the surroundings improves the accuracy of enthalpy change calculations.
- Correlation and Causation
Analysis helps distinguish between correlation and causation, a crucial aspect of scientific reasoning. Observing a relationship between two variables does not necessarily imply a causal link. Rigorous analysis, including statistical tests and control experiments, is essential for establishing whether changes in one variable directly cause changes in another. For example, in the study of catalyst effects on reaction rates, control experiments without the catalyst are necessary to confirm the catalyst’s causal role in accelerating the reaction.
These analytical tools, when applied systematically to experimental data on physical and chemical changes, reveal underlying patterns and principles governing transformations of matter. From simple physical processes like dissolution to complex chemical reactions like redox reactions, analysis provides the crucial link between observation and understanding, propelling scientific discovery and enabling the development of predictive models and technological applications.
5. Interpretation
Interpretation stands as the culminating stage in the scientific investigation of physical and chemical changes within a laboratory setting. It represents the process of transforming raw experimental data and analytical findings into meaningful scientific conclusions. Interpretation bridges the gap between empirical observations and theoretical understanding, enabling researchers to contextualize findings within existing scientific frameworks and generate new hypotheses for future investigation. In essence, interpretation provides the intellectual framework for understanding the “why” behind the observed changes, moving beyond simple description to explanation and prediction.
- Connecting Observations to Theory
Interpretation links empirical observations to established chemical principles and theories. For example, observing the evolution of a gas during a reaction, coupled with analytical data confirming the gas’s identity, could be interpreted as evidence of a decomposition reaction. Similarly, a measured increase in temperature during a reaction, combined with stoichiometric calculations, could be interpreted as evidence of an exothermic process, aligning with thermodynamic principles. This connection between observation and theory strengthens the validity of experimental findings and reinforces fundamental chemical concepts.
- Explaining Mechanisms of Change
Interpretation delves into the underlying mechanisms driving observed transformations. For instance, observing a color change during a titration, combined with an understanding of indicator chemistry, allows interpretation of the reaction’s endpoint and the stoichiometric equivalence point. In electrochemical experiments, measuring voltage changes coupled with knowledge of redox potentials allows interpretation of electron transfer processes. This mechanistic understanding provides deeper insights into the how and why of chemical and physical changes, moving beyond simple phenomenological description to a more fundamental level of explanation.
- Predicting Future Behavior
Interpretation enables predictions about the behavior of matter under different conditions. For instance, based on experimental data and established chemical principles, one can predict the solubility of a substance at different temperatures or the rate of a reaction at different concentrations. This predictive capacity is crucial for designing new materials, optimizing chemical processes, and developing technological applications. In the pharmaceutical industry, predicting drug interactions based on chemical structure and properties is vital for patient safety and effective treatment strategies.
- Formulating New Hypotheses
Interpretation often leads to the formulation of new hypotheses and research questions. Unexpected experimental results or discrepancies between observed and predicted behavior can prompt further investigation and refinement of existing theories. For example, an unexpected change in reaction rate could lead to a new hypothesis about the reaction mechanism or the role of a previously unconsidered variable. This iterative process of interpretation, hypothesis generation, and further experimentation is essential for the advancement of scientific knowledge and the refinement of our understanding of the natural world.
In the study of physical and chemical changes, interpretation acts as the critical link between experimental findings and scientific understanding. It provides the intellectual framework for explaining observed phenomena, predicting future behavior, and generating new avenues for scientific inquiry. Through careful interpretation, the data acquired in the laboratory transforms into valuable knowledge, contributing to the broader body of chemical understanding and its practical applications in various fields.
6. Safety Procedures
Safety procedures are paramount in laboratory environments investigating physical and chemical changes. These procedures mitigate risks inherent in handling chemicals and conducting experiments, protecting personnel and the surrounding environment. The direct manipulation of matter, often involving hazardous substances and energy transformations, necessitates stringent safety protocols. A failure to adhere to these protocols can result in serious consequences, ranging from minor injuries like burns or cuts to more severe incidents such as fires, explosions, or toxic exposures. The potential for unpredictable reactions and the presence of flammable, corrosive, or toxic materials underscore the critical importance of safety in this context. Real-world examples, such as improper handling of volatile organic solvents leading to fires or inadequate ventilation resulting in inhalation of toxic fumes, highlight the severe consequences of neglecting safety procedures.
Specific safety measures in the context of physical and chemical change investigations include the proper use of personal protective equipment (PPE) such as goggles, gloves, and lab coats. Appropriate ventilation systems and fume hoods are essential for preventing the accumulation of hazardous vapors. Safe storage and handling of chemicals, including proper labeling and segregation of incompatible substances, are critical. Furthermore, understanding the reactivity and potential hazards of the specific chemicals being used, as detailed in Safety Data Sheets (SDS), is crucial for developing appropriate safety protocols. Regular safety training and drills, alongside readily accessible emergency equipment like fire extinguishers and eyewash stations, are essential components of a safe laboratory environment. These measures create a multi-layered safety approach, minimizing risks and fostering a culture of safety awareness.
Effective safety procedures minimize risks and contribute to the reliability of experimental results. A safe and controlled environment allows for precise measurements and accurate observations, minimizing the potential for errors caused by accidents or uncontrolled events. The integration of safety practices into experimental design ensures the integrity of scientific investigations and fosters confidence in the validity of experimental outcomes. Ultimately, a comprehensive understanding and consistent application of safety procedures are crucial for fostering a secure and productive laboratory environment dedicated to investigating the complexities of physical and chemical changes.
7. Chemical Principles
Chemical principles provide the fundamental theoretical framework for understanding and interpreting observations made during laboratory investigations of physical and chemical changes. These principles, encompassing concepts such as stoichiometry, thermodynamics, kinetics, and equilibrium, govern the behavior of matter at the atomic and molecular level, providing a basis for predicting and explaining macroscopic phenomena observed in the lab. For instance, the law of conservation of mass, a cornerstone of chemical principles, dictates that matter is neither created nor destroyed during a chemical reaction. This principle is directly applicable in laboratory settings, where the total mass of reactants should equal the total mass of products, accounting for any gaseous products that might escape. Discrepancies in mass can be attributed to experimental error or the formation of unexpected products, prompting further investigation.
Thermodynamic principles, such as enthalpy and entropy changes, provide insights into the energy transfer associated with physical and chemical transformations. Measuring temperature changes during reactions allows for the experimental determination of enthalpy changes, providing quantitative data to classify reactions as exothermic or endothermic. Understanding these energy changes is crucial in various applications, from designing efficient combustion engines to developing effective cooling systems. Similarly, kinetic principles govern reaction rates, explaining how factors like reactant concentration, temperature, and catalysts influence the speed of a chemical transformation. Laboratory investigations exploring the effect of these factors on reaction rates provide empirical evidence supporting kinetic theories and enable the development of rate laws. Real-world examples, such as the use of catalysts in industrial processes to accelerate reactions and optimize production, demonstrate the practical significance of understanding chemical kinetics.
A deep understanding of chemical principles enables researchers to design effective experiments, interpret experimental results, and draw meaningful conclusions about the nature of physical and chemical changes. This understanding is fundamental for advancing chemical knowledge, developing new materials and technologies, and addressing real-world challenges. From the development of new pharmaceuticals to the design of sustainable energy solutions, the application of chemical principles in laboratory settings plays a crucial role in shaping scientific progress and technological innovation. Challenges such as predicting complex reaction mechanisms or understanding the behavior of matter under extreme conditions continue to drive research and deepen our understanding of these fundamental principles.
Frequently Asked Questions
This section addresses common inquiries regarding the investigation of physical and chemical changes in a laboratory setting.
Question 1: How can one differentiate between a physical and a chemical change?
A physical change alters the form or state of matter without affecting its chemical composition, while a chemical change results in the formation of new substances with different properties. Melting ice is a physical change as the water remains water, albeit in a different state. Burning wood is a chemical change as new substances, like ash and carbon dioxide, are formed.
Question 2: What are some common laboratory techniques used to study these transformations?
Common techniques include calorimetry to measure heat changes, titrations to determine concentrations, spectroscopy to analyze light interactions with matter, and chromatography to separate mixtures. These techniques provide quantitative data for characterizing transformations.
Question 3: Why is precise measurement crucial in these investigations?
Precise measurement provides quantitative data essential for accurate analysis and interpretation. It allows researchers to establish relationships between variables, determine reaction stoichiometry, and evaluate the validity of experimental findings.
Question 4: What safety precautions are essential in a chemistry lab?
Essential precautions include wearing appropriate personal protective equipment (PPE), handling chemicals according to safety data sheets (SDS), ensuring proper ventilation, and having access to emergency equipment like fire extinguishers and eyewash stations.
Question 5: How do chemical principles apply to real-world scenarios?
Chemical principles provide the basis for understanding phenomena across diverse fields. For example, thermodynamic principles are essential for designing efficient energy systems, while kinetic principles guide the development of catalysts for industrial processes.
Question 6: How can unexpected experimental results advance scientific knowledge?
Unexpected results can challenge existing theories and stimulate further investigation, leading to revised hypotheses, refined experimental designs, and a deeper understanding of the underlying principles governing transformations.
Understanding these fundamental concepts facilitates more informed experimental design, accurate data analysis, and meaningful interpretation of results.
The following sections delve further into specific experimental examples and their applications.
Conclusion
The exploration of physical and chemical changes within a controlled laboratory environment provides fundamental insights into the behavior of matter. Systematic observation, precise measurement, and rigorous experimentation, guided by established chemical principles, enable researchers to characterize transformations, elucidate reaction mechanisms, and predict the properties of substances. From the seemingly simple act of melting ice to the complex reactions driving industrial processes, the study of these changes reveals the intricate interplay of energy and matter that shapes the world around us. Understanding these principles has far-reaching implications, impacting fields ranging from materials science and medicine to environmental science and energy production.
Continued investigation into the nature of physical and chemical transformations remains essential for addressing critical challenges facing society. Developing sustainable energy sources, designing new materials with tailored properties, and understanding the complex chemical processes driving biological systems all rely on a deep understanding of these fundamental principles. Further research, coupled with technological advancements in analytical techniques and computational modeling, promises to unlock new insights into the intricate dance of atoms and molecules, paving the way for innovations that will shape the future.