Life is built upon a foundation of fundamental chemical and physical principles governing the interactions of molecules. These molecules, primarily composed of carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur, assemble into complex structures like proteins, carbohydrates, lipids, and nucleic acids. For example, the precise three-dimensional structure of a protein, dictated by the physical forces between its constituent amino acids, determines its specific biological function, be it catalyzing a metabolic reaction or transporting molecules across a cell membrane. Understanding the underlying forces that dictate these structures is key to understanding life itself.
Comprehending these foundational principles allows for advancements in numerous fields. Drug development, for instance, relies heavily on understanding how molecules interact with biological targets. Similarly, agricultural practices can be optimized by manipulating the chemical and physical properties of soil to enhance nutrient availability for plants. Historically, the study of these principles has led to breakthroughs like the elucidation of DNA’s structure, paving the way for advancements in genetic engineering and biotechnology. This understanding is essential for addressing global challenges related to health, food security, and environmental sustainability.
Further exploration will delve into specific examples of these principles in action, including the roles of covalent and non-covalent bonding in shaping macromolecular structures, the thermodynamics governing biological processes, and the kinetic principles underlying enzyme catalysis and metabolic pathways. Additionally, the impact of these principles on cellular organization and communication will be examined.
Tips for Understanding Molecular Principles in Biology
Gaining a deeper understanding of the chemical and physical principles governing biological molecules is crucial for advancements in various scientific fields. The following tips provide guidance for approaching this complex subject:
Tip 1: Focus on Foundational Concepts. Mastering fundamental concepts like atomic structure, chemical bonding (ionic, covalent, hydrogen), and intermolecular forces is essential. These concepts provide the building blocks for understanding more complex molecular interactions.
Tip 2: Visualize Molecular Structures. Employing visual aids, such as molecular models and diagrams, can significantly enhance comprehension of three-dimensional structures and their influence on molecular function.
Tip 3: Relate Structure to Function. Always consider how the specific structure of a molecule, whether a protein, carbohydrate, or lipid, directly relates to its biological role. For example, the hydrophobic nature of lipid molecules underlies their function in forming cell membranes.
Tip 4: Understand Thermodynamics and Kinetics. Biological processes are governed by thermodynamic principles (energy changes) and kinetic principles (reaction rates). A grasp of these principles is critical for analyzing metabolic pathways and enzyme activity.
Tip 5: Explore Experimental Techniques. Familiarize oneself with common experimental techniques used to study biological molecules, such as X-ray crystallography, NMR spectroscopy, and mass spectrometry. Understanding these techniques provides insight into how structural information is obtained.
Tip 6: Consider Systems Biology. Biological systems are complex networks of interacting molecules. Adopting a systems-level perspective can help illuminate how individual molecular interactions contribute to overall cellular function.
Tip 7: Stay Updated on Current Research. The field of molecular biology is constantly evolving. Staying abreast of current research findings through scientific literature and conferences is crucial for maintaining a comprehensive understanding.
By applying these tips, a more robust and comprehensive understanding of the interplay between molecular structure, function, and biological processes can be achieved.
In conclusion, the presented insights into the chemical and physical principles governing biological molecules underscore the significance of this field in advancing scientific knowledge and addressing critical challenges in various disciplines.
1. Molecular Structure
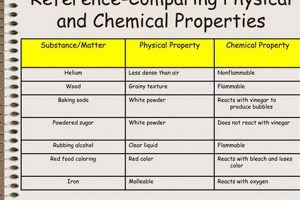
Molecular structure, the three-dimensional arrangement of atoms within a molecule, plays a pivotal role in dictating the physical and chemical properties of the molecules of life. This architecture, determined by the types of atoms present and the bonds connecting them, governs how these molecules interact with each other and their environment, ultimately underpinning all biological processes.
- Atomic Composition and Bonding:
The specific atoms within a molecule and the nature of the chemical bonds linking them (covalent, ionic, hydrogen) fundamentally influence its structure and properties. For instance, the strong covalent bonds forming the backbone of a DNA molecule contribute to its stability, while the weaker hydrogen bonds between base pairs enable replication and transcription. The differences in electronegativity between atoms also contribute to bond polarity, influencing intermolecular interactions.
- Isomerism:
Molecules with the same chemical formula but different structural arrangements, known as isomers, can exhibit dramatically different properties. Consider glucose and fructose, both C6H12O6, yet possessing distinct sweetness and metabolic fates due to variations in their atomic arrangements. Structural isomers, like these sugars, highlight how subtle changes in structure can profoundly impact biological function.
- Three-Dimensional Conformation:
Beyond the basic connectivity of atoms, the three-dimensional shape of a molecule, or its conformation, is paramount. Proteins, for instance, fold into intricate structures determined by interactions between amino acid side chains. This precise folding dictates their specific function, whether acting as enzymes, structural components, or signaling molecules. Disruptions to this conformation can lead to loss of function and disease.
- Chirality:
Many biological molecules exhibit chirality, existing as non-superimposable mirror images, like left and right hands. This property, often arising from a chiral carbon atom bonded to four different groups, has profound implications for molecular recognition. Enzymes, for example, often selectively interact with only one chiral form of a substrate, demonstrating the importance of stereochemistry in biological systems.
These facets of molecular structureatomic composition, isomerism, three-dimensional conformation, and chiralityintertwine to define the unique properties of biological molecules. This intricate relationship between structure and function forms the cornerstone of understanding the physical and chemical principles governing life processes, from enzyme catalysis to cellular communication.
2. Chemical Bonding
Chemical bonding, the attractive force holding atoms together within molecules, lies at the heart of the physical and chemical principles governing the molecules of life. The nature and strength of these bonds dictate molecular structure, stability, and reactivity, ultimately shaping the complex landscape of biological processes.
- Covalent Bonds:
Covalent bonds, formed by the sharing of electrons between atoms, constitute the fundamental structural links within biological molecules. These strong bonds form the backbone of essential biomolecules like proteins, carbohydrates, and nucleic acids. The specific arrangement of covalent bonds dictates the three-dimensional structure and, consequently, the function of these molecules. For instance, the peptide bonds linking amino acids in a protein determine its primary structure, a crucial determinant of its overall fold and activity.
- Ionic Bonds:
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. While less prevalent than covalent bonds in organic molecules, they play crucial roles in biological systems. For example, ionic interactions contribute to the stability of protein structures and mediate interactions between charged molecules, such as DNA and proteins. Furthermore, the transport of ions across cell membranes relies on the principles of electrostatic attraction and repulsion.
- Hydrogen Bonds:
Hydrogen bonds, weaker than covalent or ionic bonds, form between a hydrogen atom covalently bound to a highly electronegative atom (like oxygen or nitrogen) and another electronegative atom. These bonds play crucial roles in stabilizing the three-dimensional structures of biomolecules. The double helix of DNA is stabilized by hydrogen bonds between complementary base pairs. Similarly, hydrogen bonding contributes significantly to protein folding and enzyme-substrate interactions. Their relative weakness allows for dynamic interactions within biological systems.
- Van der Waals Forces:
Van der Waals forces represent weak, transient attractions between molecules arising from fluctuations in electron distribution. While individually weak, their cumulative effect can be substantial, particularly in large biomolecules. These forces contribute to the packing of lipid molecules in cell membranes and play a role in protein folding and molecular recognition. Their transient nature allows for flexibility and dynamic interactions in biological systems.
The interplay of these diverse chemical bondscovalent, ionic, hydrogen, and van der Waalsunderpins the intricate architecture and dynamic behavior of biological molecules. The specific combination and arrangement of these bonds determine the unique properties of each molecule, enabling the complex web of interactions essential for life. From the stable backbone of DNA to the dynamic folding of proteins, chemical bonding lies at the foundation of the molecules of life and their remarkable functions.
3. Intermolecular Forces
Intermolecular forces, the attractive or repulsive interactions between molecules, play a crucial role in shaping the structure, function, and behavior of biological systems. These forces, distinct from the stronger intramolecular forces (chemical bonds) holding atoms together within a molecule, significantly influence the physical and chemical properties of biomolecules and their interactions. Their impact extends from the stability of macromolecular structures to the dynamics of cellular processes.
Several key intermolecular forces govern the interactions of biological molecules. Hydrogen bonding, as previously discussed, contributes significantly to protein folding, DNA base pairing, and the unique properties of water. Van der Waals forces, including dipole-dipole interactions, London dispersion forces, and hydrophobic interactions, play crucial roles in protein-protein interactions, enzyme-substrate binding, and the formation of lipid membranes. The strength and specificity of these interactions dictate how biomolecules assemble, recognize each other, and perform their functions.
For instance, the hydrophobic effect, driven by the tendency of nonpolar molecules to aggregate in an aqueous environment, is fundamental to the formation of cell membranes and the folding of proteins. The lipid bilayer, the core structural component of cell membranes, forms spontaneously due to hydrophobic interactions between lipid tails, creating a barrier separating the cell’s interior from its surroundings. Similarly, the hydrophobic side chains of amino acids within a protein tend to cluster together in the protein’s core, driving the folding process and contributing to the protein’s overall stability. Understanding these intermolecular forces provides critical insights into the organization and function of biological systems.
Furthermore, the dynamic nature of intermolecular forces allows for flexibility and responsiveness in biological processes. These forces can be readily broken and reformed, enabling molecules to interact transiently, facilitating processes such as signal transduction, enzyme catalysis, and molecular transport. The ability of proteins to change conformation in response to binding partners, for example, relies on the interplay of various intermolecular forces. This dynamic interplay underlies the adaptability and responsiveness of living systems.
In summary, intermolecular forces represent a critical component of the physical and chemical principles governing the molecules of life. Their influence extends from the formation of stable macromolecular structures to the dynamic interactions essential for cellular processes. A deep understanding of these forces is therefore fundamental to comprehending the complexity and elegance of biological systems.
4. Thermodynamics
Thermodynamics, the study of energy transformations, provides a crucial framework for understanding the physical and chemical principles governing the molecules of life. Biological systems are inherently dynamic, constantly exchanging energy with their surroundings. Thermodynamic principles dictate the directionality and feasibility of biochemical reactions, influencing processes ranging from metabolism to cellular signaling. A grasp of thermodynamic concepts is essential for comprehending the intricate energy flow that sustains life.
- Enthalpy and Entropy:
Enthalpy (H) represents the heat content of a system, while entropy (S) reflects the degree of disorder. These two thermodynamic properties dictate the spontaneity of a reaction through the Gibbs free energy (G) equation: G = H – TS. Exothermic reactions (releasing heat, negative H) tend to be favorable, as do reactions that increase disorder (positive S). Protein folding, for instance, often involves a decrease in enthalpy (favorable) but also a decrease in entropy (unfavorable). The balance between these factors determines whether the overall process is spontaneous.
- Gibbs Free Energy:
Gibbs free energy (G) determines the spontaneity of a reaction at constant temperature and pressure. A negative G indicates a spontaneous reaction (exergonic), while a positive G signifies a non-spontaneous reaction (endergonic). Cellular processes often couple endergonic reactions with exergonic reactions, such as ATP hydrolysis, to drive them forward. The energy released from ATP breakdown can power energetically unfavorable reactions essential for cellular function.
- Equilibrium:
Chemical reactions proceed towards a state of equilibrium, where the rates of the forward and reverse reactions are equal. At equilibrium, G = 0. Biological systems, however, are rarely at equilibrium. They maintain a steady state by constantly exchanging energy and matter with their environment. Metabolic pathways, for example, operate far from equilibrium, continuously driving the synthesis and breakdown of biomolecules.
- Temperature and Pressure:
Temperature and pressure influence the thermodynamics of biological reactions. Enzymes, biological catalysts, exhibit optimal activity within specific temperature and pH ranges. Deviations from these optimal conditions can disrupt enzyme structure and function. Similarly, pressure changes can affect the stability and activity of biomolecules, particularly in deep-sea organisms adapted to high-pressure environments.
These thermodynamic principlesenthalpy, entropy, Gibbs free energy, equilibrium, and the influence of temperature and pressureprovide a fundamental framework for understanding the energy flow and transformations that drive biological processes. From the folding of proteins to the dynamics of metabolic pathways, thermodynamics offers essential insights into the physical and chemical principles underpinning the molecules of life.
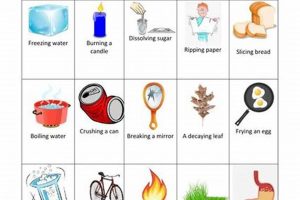
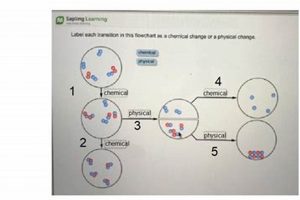
5. Chemical Reactions
Chemical reactions, the processes by which molecules interact and transform, are fundamental to the physical and chemical principles governing the molecules of life. These reactions, involving the breaking and forming of chemical bonds, underpin all biological processes, from metabolism and energy production to cellular communication and genetic replication. Understanding the dynamics and energetics of chemical reactions is essential for comprehending the intricate workings of living systems.
- Metabolic Reactions:
Metabolic reactions encompass a vast network of interconnected chemical transformations that sustain life. These reactions, including catabolic processes (breaking down molecules) and anabolic processes (building up molecules), provide the energy and building blocks necessary for cellular function. Glycolysis, the breakdown of glucose to produce ATP, exemplifies a crucial metabolic pathway. These reactions are often catalyzed by enzymes, specialized proteins that accelerate reaction rates.
- Redox Reactions:
Oxidation-reduction (redox) reactions, involving the transfer of electrons between molecules, play a central role in energy generation within cells. Photosynthesis, the process by which plants convert light energy into chemical energy, relies on a series of redox reactions to capture and store solar energy. Similarly, cellular respiration utilizes redox reactions to extract energy from glucose, ultimately producing ATP. These electron transfer processes are essential for driving the energy-requiring activities of cells.
- Polymerization Reactions:
Polymerization reactions link small molecules (monomers) together to form large macromolecules (polymers). The synthesis of proteins from amino acids, DNA from nucleotides, and polysaccharides from sugars exemplifies polymerization reactions crucial for building the essential components of cells. These reactions often involve dehydration synthesis, where a water molecule is removed as monomers are linked together. The specific sequence of monomers within a polymer dictates its unique properties and function.
- Acid-Base Reactions:
Acid-base reactions involve the transfer of protons (H+) between molecules. Maintaining proper pH balance is crucial for biological systems, as pH influences the structure and activity of biomolecules, including enzymes. Buffer systems, which resist changes in pH, play a vital role in maintaining homeostasis within cells and organisms. The bicarbonate buffer system in blood, for example, helps regulate blood pH, ensuring optimal physiological function.
These diverse chemical reaction typesmetabolic reactions, redox reactions, polymerization reactions, and acid-base reactionsrepresent fundamental processes governing the molecules of life. Their interplay and regulation dictate the intricate workings of biological systems, enabling the complex web of interactions that sustain life. From energy production to macromolecular synthesis, chemical reactions lie at the heart of the physical and chemical principles that define living organisms.
6. Kinetics
Kinetics, the study of reaction rates and mechanisms, provides crucial insights into the dynamic nature of biological systems. Understanding how quickly reactions occur and the factors influencing these rates is essential for comprehending the physical and chemical principles governing the molecules of life. Kinetics bridges the gap between thermodynamic feasibility and the actual rate at which biological processes unfold, providing a crucial link between structure, function, and time.
- Reaction Rates:
Reaction rates quantify the speed at which reactants are converted into products. These rates are influenced by factors such as reactant concentration, temperature, and the presence of catalysts. In biological systems, enzyme catalysis plays a pivotal role in accelerating reaction rates, enabling life processes to occur at biologically relevant timescales. For example, the enzyme carbonic anhydrase dramatically accelerates the conversion of carbon dioxide and water into bicarbonate, a crucial reaction for maintaining blood pH balance. Without enzymatic catalysis, many essential biological reactions would proceed too slowly to sustain life.
- Activation Energy:
Activation energy represents the energy barrier that must be overcome for a reaction to occur. Enzymes lower the activation energy, facilitating faster reaction rates without altering the overall thermodynamics of the reaction. This catalytic efficiency enables biological systems to carry out complex transformations under mild physiological conditions. The specific interactions between enzymes and their substrates, often involving transient intermolecular forces, contribute to lowering the activation energy and enhancing reaction rates.
- Reaction Mechanisms:
Reaction mechanisms describe the step-by-step sequence of elementary reactions that lead from reactants to products. Understanding these mechanisms provides crucial insights into the molecular interactions and transformations underlying biological processes. Enzyme catalysis often involves a complex interplay of substrate binding, conformational changes, and chemical bond breaking and forming. Detailed knowledge of these mechanisms allows for the development of targeted interventions, such as enzyme inhibitors, with therapeutic applications.
- Enzyme Kinetics:
Enzyme kinetics explores the relationship between enzyme activity, substrate concentration, and reaction rate. The Michaelis-Menten model, a fundamental concept in enzyme kinetics, describes the dependence of reaction rate on substrate concentration. This model, along with other kinetic analyses, allows for the determination of key enzyme parameters, such as the Michaelis constant (Km) and the maximum reaction velocity (Vmax). These parameters provide insights into enzyme efficiency and substrate affinity, essential for understanding enzyme function and regulation.
Kinetics thus illuminates the dynamic behavior of biological molecules, providing essential context for the physical and chemical principles that govern life. By understanding reaction rates, activation energy, reaction mechanisms, and enzyme kinetics, one gains a deeper appreciation for the intricate interplay between structure, function, and time in shaping the complex landscape of biological processes. This dynamic interplay, governed by the principles of kinetics, underlies the remarkable efficiency and adaptability of living systems.
7. Macromolecular Assemblies
Macromolecular assemblies, formed through the intricate interplay of numerous smaller molecules, represent a pinnacle of structural and functional complexity in biological systems. These assemblies, comprising proteins, nucleic acids, carbohydrates, and lipids, are not merely static aggregations but dynamic entities whose formation and function are governed by the fundamental physical and chemical principles discussed previously. The specific arrangement of these macromolecules within assemblies dictates their diverse roles, ranging from catalyzing biochemical reactions to maintaining cellular structure and facilitating information transfer.
The driving forces behind macromolecular assembly are the same intermolecular forces that dictate the behavior of individual molecules: hydrogen bonding, ionic interactions, van der Waals forces, and the hydrophobic effect. For example, the ribosome, a complex molecular machine responsible for protein synthesis, assembles from ribosomal RNA and numerous proteins through a precisely orchestrated interplay of these forces. Similarly, the cytoskeleton, a dynamic network of protein filaments, provides structural support and facilitates intracellular transport through the controlled assembly and disassembly of actin filaments, microtubules, and intermediate filaments. These examples highlight the importance of non-covalent interactions in mediating macromolecular assembly and function. Disruptions to these interactions, caused by mutations or changes in the cellular environment, can lead to aberrant assembly and dysfunction, underscoring their critical role in maintaining cellular integrity and function.
Furthermore, the principles of thermodynamics and kinetics govern the formation and stability of macromolecular assemblies. The assembly process must be thermodynamically favorable, with a negative Gibbs free energy change. However, the rate of assembly is dictated by kinetic factors, including the concentrations of the constituent molecules and the presence of any assembly factors that facilitate the process. Understanding these thermodynamic and kinetic constraints provides crucial insights into the regulation and dynamics of macromolecular assembly within the cellular context. The practical significance of this understanding extends to diverse fields, including drug development, materials science, and nanotechnology. By manipulating the physical and chemical principles governing macromolecular assembly, researchers can design novel materials with specific properties, develop targeted therapies that disrupt disease-associated assemblies, and engineer artificial molecular machines with tailored functionalities.
In conclusion, macromolecular assemblies represent a remarkable testament to the power of physical and chemical principles in shaping complex biological systems. The intricate interplay of intermolecular forces, thermodynamics, and kinetics governs the formation, stability, and function of these assemblies, enabling the diverse array of processes essential for life. Further research into the mechanisms of macromolecular assembly promises to deepen our understanding of cellular organization and function, opening new avenues for scientific discovery and technological advancement.
Frequently Asked Questions
This section addresses common inquiries regarding the physical and chemical principles governing the molecules of life, aiming to clarify key concepts and dispel misconceptions.
Question 1: How does the structure of a molecule determine its function in biological systems?
Molecular structure dictates function by determining the specific interactions a molecule can engage in. For instance, the precise three-dimensional arrangement of amino acids in a protein determines its active site conformation, enabling it to bind specific substrates and catalyze biochemical reactions. Similarly, the complementary base pairing in DNA, dictated by its double helix structure, enables accurate replication and transmission of genetic information.
Question 2: What is the significance of intermolecular forces in biological macromolecules?
Intermolecular forces, though weaker than covalent bonds, play crucial roles in stabilizing macromolecular structures and mediating dynamic interactions. Hydrogen bonds stabilize the secondary structure of proteins and the double helix of DNA. Hydrophobic interactions drive protein folding and the formation of lipid membranes. These forces are essential for maintaining structural integrity and enabling transient interactions necessary for cellular processes.
Question 3: How do thermodynamic principles influence biochemical reactions?
Thermodynamics dictates the spontaneity and equilibrium of biochemical reactions. Gibbs free energy (G) determines whether a reaction will occur spontaneously (negative G) or require energy input (positive G). Living organisms often couple energetically unfavorable reactions with favorable ones, like ATP hydrolysis, to drive essential processes forward.
Question 4: What role does kinetics play in understanding biological processes?
Kinetics examines reaction rates and mechanisms. While thermodynamics determines whether a reaction can occur, kinetics determines how fast it occurs. Enzymes, biological catalysts, accelerate reaction rates by lowering activation energy, enabling life processes to proceed at biologically relevant timescales.
Question 5: How does the hydrophobic effect contribute to the formation of biological structures?
The hydrophobic effect, driven by the tendency of nonpolar molecules to minimize contact with water, is crucial for the formation of cell membranes and the folding of proteins. Hydrophobic interactions drive the aggregation of lipid molecules into bilayers, forming the structural basis of cell membranes. Similarly, hydrophobic amino acid side chains tend to cluster within the core of a protein, contributing to its stable folded conformation.
Question 6: Why is understanding the principles of macromolecular assembly important?
Macromolecular assemblies, such as ribosomes and the cytoskeleton, carry out complex biological functions. Understanding the principles governing their formation, stability, and dynamics is crucial for comprehending cellular processes and developing targeted interventions. Dysregulation of macromolecular assembly can lead to various diseases, highlighting the importance of these principles in human health.
A deep understanding of these physical and chemical principles provides a fundamental basis for comprehending the complexity and elegance of life processes.
Further exploration will delve into specific examples illustrating the application of these principles in various biological contexts.
The Molecules of Life
Exploration of the physical and chemical principles governing the molecules of life reveals a profound interplay of forces shaping biological structure and function. From the fundamental principles of chemical bonding and intermolecular forces to the dynamic processes governed by thermodynamics and kinetics, these principles dictate the intricate interactions within and between biomolecules. The specific arrangement of atoms within molecules, coupled with the dynamic interplay of covalent and non-covalent interactions, determines the unique properties of proteins, carbohydrates, lipids, and nucleic acids, enabling their diverse roles in cellular processes. Furthermore, the assembly of these macromolecules into higher-order structures, such as ribosomes and membranes, highlights the importance of these principles in shaping cellular organization and function.
Continued investigation into the molecules of life and the underlying physical and chemical principles offers profound potential for advancements across scientific disciplines. Unraveling the intricate mechanisms governing molecular interactions promises not only to deepen fundamental biological understanding but also to pave the way for novel therapeutic strategies, innovative biotechnologies, and sustainable solutions to global challenges. A deeper comprehension of these fundamental principles remains crucial for advancing scientific knowledge and addressing the complex interplay of life processes.