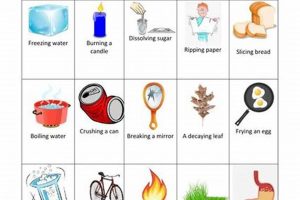
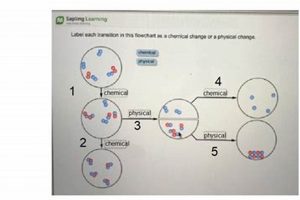
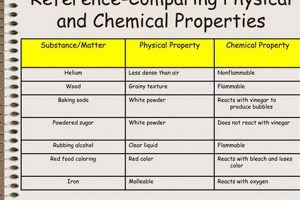
Experiments exploring transformations of matter typically involve observing alterations in properties like shape, size, and state (solid, liquid, gas), which characterize physical changes. These are contrasted with chemical changes, where new substances with different properties are formed, often evidenced by color changes, gas production, or the release or absorption of heat. A classic example involves comparing the melting of ice (physical) to the burning of a magnesium strip (chemical).
Investigating these processes provides foundational knowledge in chemistry, enabling understanding of concepts such as conservation of mass, reactivity, and the characteristics of various substances. Historically, the systematic study of such changes has been crucial to advancements in fields ranging from materials science to medicine. A strong grasp of these fundamental principles allows for the development of new materials, medicines, and technologies.
This exploration of matter’s transformations frequently involves laboratory procedures focusing on observation, measurement, and analysis. The following sections will delve into specific experimental designs, safety precautions, and the interpretation of results in these investigations. Furthermore, the discussion will extend to the broader applications of this knowledge in scientific research and technological development.
Tips for Investigating Matter Transformations
Careful observation and adherence to safety protocols are crucial for successful experimentation and accurate interpretation of results when exploring changes in matter.
Tip 1: Precise Observation: Document all initial and final states of materials. Note changes in color, texture, temperature, and state of matter. Detailed observations facilitate accurate categorization of the transformation.
Tip 2: Controlled Experiments: Isolate variables to understand their impact. Altering only one factor at a time allows for clear identification of the cause-and-effect relationship in a given change.
Tip 3: Accurate Measurement: Utilize appropriate instruments for measuring mass, volume, and temperature. Precise measurements enable quantitative analysis and support conclusions about the conservation of mass.
Tip 4: Safety First: Wear appropriate personal protective equipment, including eye protection and gloves. Handle chemicals with caution and follow established safety guidelines.
Tip 5: Waste Disposal: Adhere to proper waste disposal procedures. Different substances require specific disposal methods to minimize environmental impact and maintain laboratory safety.
Tip 6: Data Recording: Maintain a detailed laboratory notebook. Record observations, measurements, calculations, and any deviations from the experimental procedure. Clear documentation ensures reproducibility and accurate analysis.
Tip 7: Interpretation of Results: Analyze collected data to draw conclusions about the nature of the changes observed. Relate experimental findings to established scientific principles.
Adherence to these guidelines enables researchers to conduct effective investigations into the nature of physical and chemical changes, leading to accurate conclusions and a deeper understanding of the properties of matter. This foundation is essential for further exploration of complex scientific concepts and practical applications in various fields.
The subsequent sections will provide detailed examples of experiments demonstrating these transformations and offer further insights into the theoretical underpinnings of this critical area of scientific inquiry.
1. Observation
Observation forms the cornerstone of scientific inquiry, particularly within the context of investigating transformations of matter. In a laboratory setting exploring physical and chemical changes, keen observation provides the initial data upon which subsequent analysis and interpretation rely. The ability to discern subtle alterations in a substance’s properties, such as color, texture, state, or the evolution of gas, is essential for classifying the type of change occurring. For instance, observing the formation of a precipitate upon mixing two clear solutions strongly suggests a chemical change, while noting a change in volume upon heating a gas signifies a physical change. The rigor and detail of initial observations directly influence the accuracy and reliability of subsequent experimental conclusions.
Consider the classic experiment of heating sugar. Careful observation reveals the initial melting of the solid sugar crystals, indicative of a physical change. As heating continues, observation notes a color shift from white to brown, accompanied by the release of a characteristic caramel odor. These latter observations suggest a chemical change, the decomposition of sugar into new substances. Without meticulous observation, these distinct phases of transformation, and the different types of change they represent, could be easily overlooked. Accurate recording of these observations provides empirical evidence crucial for understanding the underlying processes at play.
In summary, observation acts as the primary data acquisition method in experiments exploring changes in matter. The precision and comprehensiveness of observational data directly correlate with the validity of subsequent interpretations. Developing strong observational skills is therefore paramount for researchers investigating the fundamental properties of matter and their transformations, leading to more accurate understandings of chemical and physical processes and their broader implications.
2. Measurement
Quantitative data acquisition through measurement is integral to the investigation of physical and chemical changes within a laboratory setting. Precise measurements provide empirical evidence for analysis, enabling researchers to discern patterns, draw conclusions, and formulate scientific theories. In the context of transformations of matter, measurement allows for the quantification of changes in properties like mass, volume, temperature, and pressure, providing crucial insights into the nature of these transformations.
- Mass
Measuring mass is fundamental to understanding the conservation of mass principle, a cornerstone of chemical reactions. In a closed system, the total mass of reactants equals the total mass of products, even if a chemical change alters the substances involved. Precise measurements with analytical balances verify this principle and offer insights into the stoichiometry of reactions.
- Volume
Volume measurements are particularly relevant when studying physical changes involving state transitions or the behavior of gases. Changes in temperature and pressure directly influence the volume of gases, as described by gas laws. Precise volume measurements, often using graduated cylinders or burettes, provide quantitative data to analyze these relationships.
- Temperature
Temperature changes often accompany both physical and chemical changes. The melting point of a solid, a physical property, is determined through precise temperature measurement. Similarly, the heat evolved or absorbed during a chemical reaction (enthalpy change) requires accurate temperature monitoring, often using thermometers or temperature probes.
- Pressure
Pressure measurements are crucial for understanding gas behavior and reactions involving gases. The pressure of a gas, measured using manometers or pressure sensors, relates directly to its volume, temperature, and the number of gas molecules present. Monitoring pressure changes provides insights into the dynamics of gas-phase reactions and physical processes like evaporation and condensation.
The interplay of these measured quantities provides a comprehensive understanding of transformations in matter. Correlating changes in mass, volume, temperature, and pressure allows researchers to distinguish between physical and chemical changes, analyze reaction kinetics, and formulate predictive models. Accurate measurement therefore serves as a critical bridge between observation and interpretation in the investigation of matter’s properties and its transformations.
3. Experimentation
Experimentation serves as the cornerstone of scientific inquiry within a “physical and chemical changes lab” context. It provides a structured framework for manipulating variables, observing outcomes, and gathering empirical data to test hypotheses related to matter transformations. Controlled experiments, characterized by the manipulation of a single independent variable while holding others constant, establish cause-and-effect relationships. For example, heating a known mass of ice while monitoring temperature reveals the relationship between heat input and phase transitions. Systematic variation of experimental conditions, such as changing the concentration of reactants, enables researchers to explore reaction rates and mechanisms. Experimentation allows for the practical demonstration of abstract scientific principles, connecting theoretical understanding to tangible observations. Consider the reaction between baking soda and vinegar: careful experimentation reveals gas evolution (carbon dioxide), demonstrating a chemical change with observable consequences.
The design and execution of experiments within this context necessitate careful consideration of various factors. Control groups provide a baseline for comparison, ensuring observed changes are attributable to manipulated variables. Replicating experiments ensures data reliability and reduces the impact of random errors. Careful measurement and observation are crucial for collecting accurate data, while meticulous record-keeping facilitates reproducibility and analysis. Addressing potential sources of error and implementing appropriate safety measures are paramount for generating reliable results and maintaining a secure laboratory environment. Analyzing experimental outcomes allows researchers to validate hypotheses, refine scientific models, and deepen understanding of fundamental chemical and physical principles. For instance, experiments investigating the rusting of iron demonstrate the role of oxygen and water in this chemical change, informing corrosion prevention strategies.
In conclusion, experimentation is integral to understanding physical and chemical changes. Well-designed experiments, coupled with rigorous analysis, translate abstract concepts into tangible observations, bridging theory and practice. This process of controlled investigation provides insights into the fundamental properties of matter, the nature of chemical reactions, and the practical applications of these principles in various fields. The challenges of experimental design and interpretation emphasize the importance of critical thinking and meticulous technique in scientific research related to matter transformations.
4. Analysis
Analysis within the context of a physical and chemical changes lab represents the crucial bridge between observation and interpretation. It involves systematically examining collected data measurements of mass, volume, temperature, and observations of color changes, gas evolution, or precipitate formation to determine the nature of the transformation that occurred. Analysis requires applying scientific principles and reasoning. For example, a mass change after heating an open container suggests a chemical reaction involving a gas, while a constant mass despite a phase change confirms a physical transformation. Quantitative analysis, using stoichiometry in chemical reactions, allows determination of reactant and product relationships. Qualitative analysis, like observing a color change, signifies formation of new substances.
The importance of analysis lies in its ability to reveal underlying processes driving observed changes. Consider a metal reacting with an acid: analysis of gas produced identifies it as hydrogen, revealing the reaction type as a single displacement reaction. Analyzing temperature changes quantifies heat absorbed or released, indicating whether the reaction is endothermic or exothermic. These analytical insights are fundamental to understanding chemical reactivity and predicting outcomes. Furthermore, analysis plays a critical role in quality control, material science, and environmental monitoring. Analyzing the composition of a mixture identifies contaminants, guiding purification processes. In environmental science, analysis reveals pollutants and informs remediation strategies. These practical applications demonstrate the far-reaching implications of analysis beyond the laboratory setting.
Effective analysis requires accurate data, rigorous methodology, and awareness of potential errors. Statistical analysis helps determine data reliability and significance. Understanding experimental limitations and systematic errors strengthens interpretation validity. Challenges may arise in complex systems with multiple interacting variables, necessitating advanced analytical techniques. However, mastering analytical skills remains essential for researchers, fostering critical thinking and problem-solving abilities crucial for advancements in scientific understanding and its technological applications. The ability to analyze data from investigations of matter transformations empowers researchers to unravel complexities of the material world, contributing to innovations across scientific disciplines.
5. Interpretation
Interpretation within the context of a physical and chemical changes lab signifies the process of assigning meaning to the data collected through observation, measurement, and experimentation. It involves drawing conclusions about the nature of the transformations observed, classifying them as either physical or chemical changes, and explaining the underlying scientific principles governing these changes. Accurate interpretation relies on a thorough understanding of scientific concepts, such as the conservation of mass, the characteristics of different states of matter, and the indicators of chemical reactions. For instance, observing a change in state without a change in composition, like ice melting into water, is interpreted as a physical change. Conversely, the formation of a new substance with different properties, as seen in the rusting of iron, indicates a chemical change. Cause-and-effect relationships are central to interpretation, linking observed phenomena to specific processes. The evolution of gas during a reaction, for example, can be interpreted as evidence of a chemical change producing a gaseous product.
The importance of accurate interpretation extends beyond simply classifying observed changes. It allows for the development of predictive models, enabling researchers to anticipate the outcomes of future experiments or real-world scenarios. Understanding the factors that influence reaction rates, for example, allows for optimizing reaction conditions in industrial processes. Correctly interpreting the results of a materials testing experiment can inform the selection of appropriate materials for specific applications, from building bridges to designing medical implants. Moreover, interpretation plays a crucial role in troubleshooting and problem-solving. If an expected outcome is not observed, careful interpretation of the experimental data can help identify potential sources of error or unforeseen variables influencing the results. A classic example is interpreting the results of a flame test: different colors correspond to specific metal ions, enabling identification of unknown substances.
Challenges in interpretation can arise from complex systems or ambiguous data. Distinguishing between a mixture and a new compound, for instance, can require sophisticated analytical techniques and careful interpretation of spectral data. The presence of multiple simultaneous transformations can complicate analysis, requiring careful experimental design and data interpretation to isolate individual processes. Despite these challenges, developing strong interpretive skills is paramount for researchers. It fosters critical thinking, problem-solving abilities, and the capacity to connect experimental findings to broader scientific understanding. Ultimately, the ability to accurately interpret the results of investigations involving matter transformations contributes significantly to advancements in scientific knowledge and its practical applications across various fields.
6. Safety
Safety is paramount in environments involving the investigation of physical and chemical changes. Laboratories exploring these transformations frequently utilize potentially hazardous substances and equipment, necessitating stringent safety protocols to mitigate risks and ensure a secure working environment. Negligence in safety procedures can lead to accidents, injuries, and environmental contamination. Therefore, a comprehensive understanding of safety principles and their rigorous application is crucial for all individuals involved in such scientific endeavors.
- Personal Protective Equipment (PPE)
Appropriate PPE acts as the first line of defense against potential hazards. Eye protection (goggles or safety glasses) shields against chemical splashes and flying debris. Gloves protect hands from corrosive substances and prevent skin absorption of harmful chemicals. Lab coats or aprons provide an additional layer of protection for clothing and skin. Proper footwear, such as closed-toe shoes, minimizes the risk of foot injuries from dropped objects or chemical spills. The selection and use of PPE should align with the specific hazards present in the laboratory environment.
- Chemical Handling
Safe chemical handling procedures are crucial for preventing accidents and minimizing exposure risks. Chemicals should be clearly labeled and stored appropriately, segregating incompatible substances. Using fume hoods when working with volatile or toxic chemicals limits exposure to hazardous vapors. Accurate measurement and careful transfer of chemicals minimize the risk of spills. Knowledge of Safety Data Sheets (SDS) provides essential information about chemical properties, hazards, and appropriate handling procedures. Waste disposal should follow established protocols, ensuring environmental protection and preventing cross-contamination.
- Equipment Operation
Operating laboratory equipment safely requires proper training and adherence to established procedures. Heating devices, such as Bunsen burners and hot plates, should be used with caution, avoiding flammable materials and ensuring proper ventilation. Glassware should be inspected for cracks or chips before use, and heated glassware should be handled with appropriate tongs or gloves. Electrical equipment must be properly grounded to prevent electrical shocks. Regular maintenance and inspection of equipment ensure optimal functionality and minimize the risk of malfunctions.
- Emergency Procedures
Familiarity with emergency procedures is crucial for responding effectively to accidents. Knowing the location and operation of safety equipment, such as fire extinguishers, eyewash stations, and safety showers, enables immediate action in emergencies. Established evacuation routes and assembly points ensure orderly evacuation in case of fire or other major incidents. Regular safety drills reinforce these procedures and promote a culture of preparedness.
These facets of laboratory safety are inextricably linked, forming a comprehensive safety framework. Adherence to these protocols creates a secure environment for investigating physical and chemical changes. Integrating safety practices into every aspect of laboratory work, from experimental design to data analysis, minimizes risks and fosters a culture of responsible scientific inquiry. This emphasis on safety not only protects individuals but also ensures the integrity of scientific research, fostering trust and enabling further exploration of matter’s transformations.
Frequently Asked Questions
This section addresses common inquiries regarding the investigation of physical and chemical changes in a laboratory setting. Clarity on these fundamental concepts is essential for safe and effective experimentation.
Question 1: How can one differentiate between a physical and a chemical change?
A physical change alters a substance’s form without affecting its composition, exemplified by changes in state (solid, liquid, gas) or shape. A chemical change, however, results in the formation of new substances with different properties, often indicated by color changes, gas production, or temperature changes.
Question 2: Is the burning of wood a physical or chemical change?
Burning wood is a chemical change. Combustion reactions produce new substances, ash and gases, with different compositions than the original wood and oxygen.
Question 3: Why is the melting of ice considered a physical change?
Melting ice transitions water from solid to liquid form. The chemical composition, H2O, remains the same in both states, classifying this as a physical change.
Question 4: What indicates a chemical reaction has occurred?
Several observations suggest a chemical reaction: color change, gas formation (bubbles), temperature change (release or absorption of heat), formation of a precipitate (solid from two solutions), or a change in odor.
Question 5: Why is safety crucial in experiments involving physical and chemical changes?
Many experiments involve potentially hazardous substances or equipment. Prioritizing safety, including using proper protective equipment and following established procedures, minimizes risks and ensures a secure experimental environment.
Question 6: What is the significance of a control group in these experiments?
A control group provides a baseline for comparison. It undergoes all experimental steps except for the independent variable manipulation, allowing isolation of that variable’s effect on the observed outcome.
Understanding these fundamental distinctions and safety considerations is crucial for conducting effective and meaningful investigations into the nature of matter transformations. A thorough grasp of these principles enables more nuanced experimental design, analysis, and interpretation.
Further sections will delve into specific experimental examples and explore the broader implications of understanding physical and chemical changes.
Conclusion
Investigations into physical and chemical changes provide fundamental insights into the nature of matter and its transformations. Careful observation, precise measurement, and controlled experimentation are crucial for distinguishing between these changes. Analysis and interpretation of experimental data, guided by established scientific principles, deepen understanding of underlying processes. Prioritizing safety throughout experimental procedures ensures a secure environment for exploration and discovery. From the melting of ice to the burning of magnesium, exploring these transformations unveils fundamental principles governing the material world.
Continued exploration of physical and chemical changes holds significant implications for advancements across scientific disciplines. A deeper understanding of these transformations promises to drive innovation in materials science, medicine, and environmental science. Rigorous investigation in controlled laboratory settings remains essential for unraveling the complexities of matter and its interactions, paving the way for future technological advancements and a more comprehensive understanding of the universe.