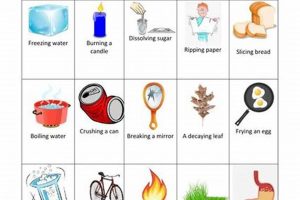
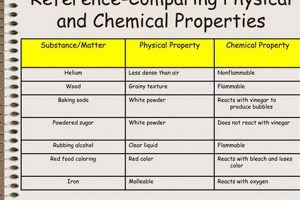
Scientific investigations often involve observing and categorizing transformations in matter. These transformations are broadly classified as either physical or chemical changes. Physical changes alter the form or appearance of a substance without affecting its composition, such as melting ice into water. Chemical changes, on the other hand, result in new substances with different properties, like iron rusting to form iron oxide. Laboratory settings provide controlled environments to study these transformations, allowing for precise measurements and observations.
Understanding the distinction between these changes is fundamental to numerous scientific disciplines, including chemistry, physics, and materials science. This knowledge is crucial for developing new materials, understanding natural processes like weathering and digestion, and even in everyday applications like cooking. Historically, the study of these changes has been instrumental in advancing our understanding of the nature of matter and the laws governing its behavior. From alchemists’ early experiments to modern chemical synthesis, the exploration of transformations has shaped scientific progress.
This exploration typically encompasses topics such as identifying indicators of chemical reactions, observing energy changes associated with transformations, and understanding the underlying molecular mechanisms at play. Examining specific examples, both commonplace and complex, will further illuminate the differences between physical and chemical processes and demonstrate the significance of controlled laboratory environments for scientific investigation.
Tips for Investigating Physical and Chemical Changes
Careful observation and methodical experimentation are crucial for accurately distinguishing between physical and chemical transformations. The following tips provide guidance for conducting effective investigations.
Tip 1: Document Initial Observations Thoroughly: Note the initial state of the substance, including its color, texture, odor, and temperature. Detailed initial observations provide a baseline for comparison after any potential transformation.
Tip 2: Control the Experimental Environment: Conduct experiments in a controlled laboratory setting to minimize external influences that could confound results. Maintaining consistent temperature, pressure, and lighting conditions ensures reliable data.
Tip 3: Look for Indicators of Chemical Change: Evidence of a chemical change may include the formation of a precipitate, gas evolution, a color change, a noticeable temperature change (indicating energy transfer), or a change in odor.
Tip 4: Consider Energy Transfer: Chemical reactions often involve energy transfer in the form of heat absorption (endothermic reactions) or heat release (exothermic reactions). Monitor temperature changes throughout the experiment.
Tip 5: Test for Reversibility: Physical changes are often easily reversible, whereas chemical changes are typically not. Attempting to reverse the observed transformation can provide further evidence for its classification.
Tip 6: Consider Changes in Composition: Chemical changes result in the formation of new substances with different chemical compositions. Analytical techniques can confirm changes in composition and distinguish between physical and chemical transformations.
Tip 7: Replicate Experiments: Repeat experiments multiple times to ensure reproducibility and validate observations. Consistent results strengthen the reliability of conclusions.
By adhering to these guidelines, investigations into transformations of matter can be conducted with greater accuracy, leading to a deeper understanding of the underlying principles governing physical and chemical changes. These practical tips facilitate both qualitative and quantitative analysis, providing a robust framework for scientific exploration.
These experimental findings can then be analyzed to draw conclusions about the nature of the transformations observed and their broader implications within the scientific context being studied.
1. Observations
Observations form the cornerstone of investigating physical and chemical changes within a laboratory setting. Careful and meticulous observation allows scientists to gather qualitative and quantitative data crucial for distinguishing between these two fundamental types of transformations. The nature of the changes observed, whether a change in color, the formation of a precipitate, gas evolution, or a temperature fluctuation, provides essential clues about the underlying processes. For example, observing the formation of bubbles upon mixing two solutions suggests a chemical reaction producing a gas, while observing a change in state, like ice melting into water, indicates a physical change. The precise nature of these observations color change from blue to green, precipitate appearing cloudy white, gas evolving rapidly, temperature increasing by 5C provides specific details about the transformation.
Quantitative observations, such as measuring the mass of reactants and products, the volume of gas produced, or the temperature change during a reaction, provide numerical data that can be analyzed to determine the extent and nature of the change. The ability to make accurate and objective observations is a critical skill in scientific investigation. Using calibrated instruments, maintaining consistent experimental conditions, and minimizing subjective interpretation ensures the reliability and validity of observations. For instance, precisely measuring the mass of magnesium ribbon before and after combustion in a crucible allows for the determination of the mass of oxygen reacted. Coupled with qualitative observations of the bright white light produced and the formation of white magnesium oxide powder, a comprehensive understanding of this chemical change emerges.
Systematic observation, combined with rigorous experimental design and controlled laboratory conditions, enables scientists to draw meaningful conclusions about the nature of the transformations studied. Detailed records of both qualitative and quantitative observations, including photographs, videos, and precise measurements, facilitate accurate interpretation and communication of experimental findings. This observational data provides the empirical evidence necessary for developing and refining scientific models that describe physical and chemical changes at a molecular level. The challenges lie in ensuring objectivity in observations, minimizing errors in measurement, and recognizing potential biases in interpretation. Addressing these challenges through established scientific methodologies ensures that observations contribute significantly to our understanding of the fundamental principles governing the behavior of matter.
2. Measurements
Quantitative measurements are essential for characterizing physical and chemical changes in a laboratory setting. Precise measurements provide objective data that allows for detailed analysis and differentiation between these transformations. They enable the quantification of changes in mass, volume, temperature, and other relevant properties, providing insights into the nature and extent of the transformation. These measurements form the basis for drawing conclusions about the processes being studied.
- Mass
Changes in mass are a key indicator in chemical reactions. Precise measurement of reactants and products using analytical balances allows for the determination of mass changes during a reaction. This data is crucial for verifying the law of conservation of mass and understanding stoichiometry. For example, measuring the mass of magnesium before and after burning in oxygen demonstrates the increase in mass due to the formation of magnesium oxide.
- Volume
Volume measurements are important for reactions involving gases. Using calibrated glassware like graduated cylinders and burets, changes in gas volume during a reaction can be quantified. This is particularly relevant for studying gas laws and reaction stoichiometry involving gaseous reactants or products. For instance, the volume of hydrogen gas produced during the reaction of a metal with an acid can be measured to determine the reaction rate.
- Temperature
Temperature changes often accompany chemical reactions, reflecting energy transfer. Thermometers or temperature probes provide precise temperature measurements, allowing for the classification of reactions as exothermic (heat-releasing) or endothermic (heat-absorbing). Measuring the temperature change during a neutralization reaction between an acid and a base reveals the exothermic nature of the process.
- Other Physical Properties
Beyond mass, volume, and temperature, other measurable physical properties, such as density, conductivity, and pH, provide further insights into the nature of the transformation. Changes in these properties can be indicative of a chemical change. For example, measuring the pH change during a reaction between an acid and a base reveals the formation of a salt and water.
These various measurements, taken with precision and accuracy using calibrated instruments, provide a comprehensive quantitative picture of the transformation. This data, combined with qualitative observations, allows for a complete characterization of the process, distinguishing between physical and chemical changes and revealing the underlying principles governing the behavior of matter. Analyzing these measurements within the context of established scientific theories and models enhances understanding of the observed phenomena.
3. Reversibility
Reversibility serves as a critical criterion for distinguishing between physical and chemical changes in laboratory investigations. A physical change is generally easily reversible, meaning the original substance can be recovered through simple physical processes. For example, melting ice into water is a physical change because the water can be frozen back into ice, returning it to its original state. Similarly, dissolving salt in water is a physical change because the salt can be recovered by evaporating the water. The ease of reversing these transformations demonstrates that the composition of the substance remains unchanged; only its physical state or form is altered. Chemical changes, conversely, are typically not easily reversible. Once a chemical reaction occurs, new substances with different properties are formed, making it difficult or impossible to return to the original reactants through simple physical means. For instance, burning wood produces ash and smoke. It is not possible to simply recombine the ash and smoke to obtain the original wood. This irreversibility stems from the fundamental alteration in the chemical composition of the wood during combustion.
The concept of reversibility has practical implications in numerous applications. In materials science, understanding the reversibility of phase transitions is crucial for designing materials with specific properties. For example, shape memory alloys exhibit reversible phase transformations that allow them to return to their original shape after deformation. In chemical engineering, the reversibility of chemical reactions is a key consideration in process design and optimization. Reversible reactions can reach a state of equilibrium, where the rates of the forward and reverse reactions are equal. Manipulating reaction conditions, such as temperature and pressure, can shift the equilibrium to favor the desired product. Furthermore, the concept of reversibility is integral to environmental science, where understanding the reversibility of processes like pollution is critical for developing remediation strategies. While some pollutants can be removed through physical processes (filtration, sedimentation), others require chemical transformations to detoxify them.
Assessing reversibility requires careful observation and experimentation. While physical changes are generally easily reversible, some may require specific conditions. For instance, condensing water vapor back into liquid water requires cooling. The challenge lies in differentiating processes that are truly irreversible from those that are simply difficult to reverse in a laboratory setting. Advanced analytical techniques, such as spectroscopy and chromatography, can confirm whether a chemical change has occurred by identifying the presence of new substances. Understanding the interplay between reversibility, experimental limitations, and analytical techniques is essential for drawing accurate conclusions about the nature of transformations observed in the lab. This knowledge ultimately strengthens the understanding of fundamental chemical and physical principles.
4. Energy Transfer
Energy transfer is a fundamental aspect of both physical and chemical changes, providing crucial insights into the nature of these transformations. In laboratory settings, observing and quantifying energy changes offer valuable data for characterizing and distinguishing between physical and chemical processes. Analysis of energy transfer during these changes elucidates the underlying mechanisms and principles governing the behavior of matter.
- Exothermic Reactions
Chemical reactions that release energy into the surroundings are classified as exothermic. This energy release often manifests as heat, causing a temperature increase in the system and its surroundings. Combustion reactions, such as burning wood or magnesium, are classic examples of exothermic processes, releasing significant heat and light. In laboratory settings, the heat released during exothermic reactions can be measured using calorimetry, providing quantitative data on the energy changes involved.
- Endothermic Reactions
Endothermic reactions absorb energy from the surroundings, typically resulting in a temperature decrease. These reactions require an input of energy to proceed. Dissolving ammonium nitrate in water is an example of an endothermic process, causing the solution to cool. In the lab, endothermic changes can be observed by monitoring temperature changes or measuring the energy required to maintain a constant temperature.
- Physical Changes and Energy
Physical changes also involve energy transfer, although they do not result in the formation of new substances. Phase transitions, such as melting, boiling, and evaporation, are endothermic processes, requiring energy input to overcome intermolecular forces. Conversely, condensation and freezing are exothermic, releasing energy as intermolecular forces strengthen. Precise measurements of energy changes during phase transitions provide information about the specific heat capacity and latent heat of substances.
- Energy and Reaction Rates
The energy required to initiate a chemical reaction is called the activation energy. Understanding energy transfer in reactions provides insights into reaction rates. Increasing the temperature typically increases reaction rates by providing more energy for reactant molecules to overcome the activation energy barrier. Catalysts can lower activation energy, thereby increasing reaction rates without being consumed in the reaction. Laboratory experiments manipulating temperature and catalyst presence demonstrate the interplay between energy transfer and reaction kinetics.
The study of energy transfer in laboratory settings is crucial for understanding the driving forces behind physical and chemical changes. By carefully measuring and analyzing energy changes, scientists gain a deeper understanding of reaction mechanisms, thermodynamic principles, and the relationship between energy and the behavior of matter. This knowledge is essential for a wide range of applications, from designing efficient energy storage systems to developing new materials and understanding biological processes.
5. Compositional Changes
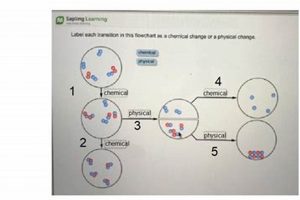
Compositional changes are central to understanding the distinction between physical and chemical changes in laboratory settings. While physical changes alter the form or state of a substance without affecting its underlying composition, chemical changes inherently involve the formation of new substances with different compositions. Analyzing compositional changes provides definitive evidence for chemical transformations and offers insights into the nature of the reactions taking place.
- Chemical Reactions and New Substances
Chemical reactions inherently involve the rearrangement of atoms and the formation of new chemical bonds. This rearrangement results in the creation of substances with compositions different from the initial reactants. For example, the reaction between hydrogen and oxygen to form water involves a compositional change from the diatomic elements to a compound with a 2:1 ratio of hydrogen to oxygen atoms. Laboratory analysis, such as spectroscopy or chromatography, can confirm the formation of new substances by identifying their unique chemical signatures.
- Elemental Analysis
Techniques like elemental analysis provide precise information about the elemental composition of substances. By comparing the elemental composition of reactants and products, chemical changes can be definitively identified. For instance, combusting magnesium in air forms magnesium oxide. Elemental analysis would reveal the change in composition from pure magnesium to a compound containing both magnesium and oxygen.
- Indicators of Compositional Change
Several observable indicators in a laboratory setting can suggest compositional changes. The formation of a precipitate, gas evolution, a distinct color change, or a significant temperature change can all be indicative of a chemical reaction and therefore a change in composition. While these observations are not always conclusive, they provide strong evidence for chemical transformations, prompting further investigation using analytical techniques.
- Conservation of Mass
While chemical reactions alter the composition of substances, the total mass of the system remains constant. This principle, known as the law of conservation of mass, states that matter is neither created nor destroyed in chemical reactions. In a lab setting, careful measurements of the mass of reactants and products demonstrate this principle. While the composition may change, the total mass before and after a chemical reaction will remain the same, demonstrating the rearrangement of atoms into new configurations.
Analyzing compositional changes is crucial for confirming chemical reactions and understanding the fundamental principles governing chemical transformations. Laboratory techniques provide the necessary tools to identify these changes, enabling a deeper understanding of the nature of matter and its interactions. By combining observational data with quantitative measurements of compositional changes, researchers can elucidate reaction mechanisms, predict reaction outcomes, and develop new applications based on chemical transformations. This understanding is essential for advancements in various scientific fields, from materials science to medicine and environmental science.
6. Controlled Environment
Controlled environments are essential for the accurate study of physical and chemical changes. Laboratories provide such settings, enabling researchers to minimize external influences and isolate the specific transformations under investigation. This control allows for precise manipulation of variables like temperature, pressure, and reactant concentrations, facilitating reproducible results and a deeper understanding of the underlying principles governing matter transformations. A controlled environment ensures that observed changes are attributable to the intended experimental manipulations rather than extraneous factors.
- Variable Manipulation
Laboratory environments allow for precise control and manipulation of experimental variables. Temperature, pressure, and reactant concentrations can be adjusted and maintained at specific values to investigate their influence on physical and chemical changes. This controlled manipulation ensures that observed effects are directly related to the altered variable, facilitating the study of reaction kinetics, equilibrium conditions, and thermodynamic properties. For example, studying the rate of a chemical reaction at different temperatures allows for the determination of the activation energy.
- Elimination of Extraneous Influences
Controlled laboratory settings minimize interference from external factors that could confound experimental results. By controlling factors like humidity, light exposure, and atmospheric composition, researchers can isolate the specific transformation being studied and ensure that observed changes are solely attributable to the experimental conditions. This is crucial for obtaining accurate data and drawing valid conclusions about the nature of the changes. For instance, studying oxidation reactions in an inert atmosphere prevents unwanted side reactions with atmospheric oxygen.
- Reproducibility and Reliability
Controlled environments promote reproducibility of experiments. By maintaining consistent experimental conditions across multiple trials, researchers can ensure that results are reliable and not influenced by uncontrolled variations. This reproducibility is crucial for validating experimental findings and building a robust understanding of scientific phenomena. Replicating experiments under identical controlled conditions strengthens the validity of observed trends and relationships.
- Safety and Containment
Laboratory settings provide a safe and controlled environment for handling potentially hazardous substances or reactions. Fume hoods and other safety equipment minimize exposure to harmful chemicals or byproducts. Containment measures prevent environmental contamination and ensure the safety of researchers. This controlled setting allows for the investigation of reactions that would be too dangerous to conduct in uncontrolled environments. For instance, handling corrosive acids or flammable materials requires the controlled environment of a laboratory.
The controlled environment of a laboratory is essential for the systematic investigation of physical and chemical changes. By manipulating variables, minimizing extraneous influences, promoting reproducibility, and ensuring safety, laboratory settings provide the necessary conditions for rigorous scientific inquiry. This control enables researchers to isolate, observe, and measure transformations with precision, leading to a deeper understanding of the fundamental principles governing the behavior of matter. The insights gained from controlled laboratory experiments contribute to advancements in diverse fields, from materials science and medicine to environmental science and engineering.
7. Experimental Design
Experimental design plays a crucial role in investigating physical and chemical changes within a laboratory setting. A well-considered experimental design ensures that investigations are conducted systematically, minimizing bias and maximizing the validity and reliability of results. Careful planning of experimental procedures, including variable control, data collection methods, and analysis techniques, is essential for drawing meaningful conclusions about the nature of the transformations being studied. Cause-and-effect relationships between experimental manipulations and observed changes can only be confidently established through rigorous experimental design. For instance, when investigating the effect of temperature on reaction rate, a well-designed experiment would involve systematically varying the temperature while holding all other reaction conditions constant. This isolates the effect of temperature and allows for a clear determination of its influence on the reaction rate. Without a robust experimental design, confounding factors can obscure the true relationship between variables, leading to inaccurate or misleading conclusions.
Several key elements contribute to a robust experimental design in the context of studying physical and chemical changes. A clear research question or hypothesis guides the experimental design, focusing the investigation on specific aspects of the transformation. Careful selection of appropriate materials and equipment ensures accurate measurements and observations. Controlling variables, such as temperature, pressure, and reactant concentrations, is essential for isolating the effects of specific manipulations. Implementing appropriate safety measures is paramount for protecting researchers and ensuring the integrity of the experiment. Replicating experiments multiple times allows for the assessment of variability and increases confidence in the results. Appropriate data analysis techniques, such as statistical analysis and graphical representation, facilitate the interpretation of experimental findings and the drawing of valid conclusions. For example, when investigating the decomposition of hydrogen peroxide, using a catalyst and measuring the volume of oxygen gas produced at regular time intervals provides quantitative data that can be analyzed to determine the reaction rate and the effect of the catalyst. Careful documentation of all experimental procedures and results is crucial for transparency and reproducibility.
Understanding the principles of experimental design is fundamental for conducting meaningful scientific investigations into physical and chemical changes. A well-designed experiment enables researchers to isolate and study specific transformations, minimizing the influence of extraneous factors and maximizing the reliability of results. This understanding leads to more accurate interpretations of experimental data, facilitating the development of scientific models and theories that describe the behavior of matter. Challenges in experimental design often involve balancing the desire for highly controlled conditions with the need to ensure that experiments are representative of real-world phenomena. Addressing these challenges requires careful consideration of the research question, the limitations of available resources, and the potential impact of uncontrolled variables. Ultimately, a robust experimental design is essential for advancing scientific knowledge and understanding the intricate processes governing physical and chemical transformations in the world around us. The insights gained from well-designed experiments contribute to advancements in various fields, from materials science and medicine to environmental science and engineering.
Frequently Asked Questions about Physical and Chemical Changes
This section addresses common queries regarding the differentiation and investigation of physical and chemical changes within a laboratory context.
Question 1: How can one definitively determine if a change is physical or chemical?
While observable indicators like color change or gas evolution can suggest a chemical change, definitive confirmation requires analyzing compositional changes. Techniques like spectroscopy or chromatography identify new substances formed during a reaction, providing conclusive evidence of a chemical transformation. Physical changes alter only the form or state, leaving the composition unchanged.
Question 2: What is the significance of a controlled environment in these investigations?
A controlled laboratory environment minimizes external influences, allowing researchers to isolate specific transformations and manipulate variables precisely. This control ensures that observed changes are directly attributable to experimental manipulations, enhancing the reliability and reproducibility of results. It also allows for the safe handling of potentially hazardous materials or reactions.
Question 3: Why is accurate measurement crucial for studying these transformations?
Precise measurements of mass, volume, temperature, and other relevant properties provide quantitative data essential for characterizing the extent and nature of the change. These measurements enable a deeper understanding of the underlying principles governing the transformation, such as stoichiometry, energy transfer, and reaction kinetics.
Question 4: How does the concept of reversibility help distinguish between the two types of changes?
Physical changes are generally easily reversible, meaning the original substance can be recovered through simple physical processes. Chemical changes, however, are typically irreversible due to the formation of new substances with different properties. While some physical changes may require specific conditions for reversal, chemical changes involve fundamental alterations in composition, making reversal difficult or impossible.
Question 5: What role does energy transfer play in these changes?
Energy transfer is a fundamental aspect of both physical and chemical changes. Exothermic chemical reactions release energy, often as heat, while endothermic reactions absorb energy. Physical changes, like phase transitions, also involve energy transfer. Analyzing energy changes provides insights into reaction mechanisms and thermodynamic properties.
Question 6: How does experimental design impact the reliability of results?
A well-designed experiment ensures that investigations are conducted systematically, minimizing bias and maximizing the validity of results. Controlling variables, replicating experiments, and employing appropriate data analysis techniques are crucial for drawing accurate conclusions about the nature of the observed transformations.
Understanding these fundamental concepts regarding physical and chemical changes, coupled with rigorous laboratory practices, is essential for advancing scientific knowledge in various disciplines.
Further exploration may involve investigating specific examples of physical and chemical changes in greater detail, delving into specific analytical techniques, or examining the practical applications of this knowledge in diverse fields.
Conclusion
Laboratory investigations of physical and chemical changes provide essential insights into the fundamental nature of matter. Careful observation, precise measurement, and controlled experimentation enable differentiation between transformations that alter form without changing composition (physical changes) and those that result in new substances (chemical changes). Analysis of energy transfer, reversibility, and compositional changes provides conclusive evidence for classifying transformations. Rigorous experimental design, including variable control and meticulous data collection, is crucial for drawing valid conclusions.
Continued exploration of physical and chemical changes through refined experimental techniques and advanced analytical methods promises to deepen understanding of the intricate processes governing the behavior of matter. This knowledge underpins advancements in diverse scientific disciplines, from developing novel materials to understanding complex biological systems and addressing critical environmental challenges. Further investigation and application of these principles are essential for continued scientific progress.