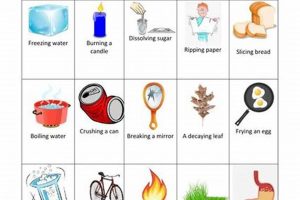
Laboratory investigations focused on differentiating between transformations of matter provide crucial insights into the nature of substances. A classic experiment might involve observing the burning of a magnesium ribbon (a chemical alteration resulting in a new substance, magnesium oxide) and contrasting it with the melting of ice (a physical shift where water changes state but retains its chemical identity). These practical exercises often include observations of changes in properties like color, odor, temperature, and the production of gas or light.
Understanding the distinction between alterations that affect only physical properties and those that result in new substances is fundamental to chemistry. This knowledge has far-reaching implications, from developing new materials and medicines to comprehending geological processes and even cooking. Historically, the development of this understanding was crucial to advancing the field of chemistry from alchemy to a true science, enabling systematic investigation and prediction of material behavior.
This foundational knowledge serves as a springboard for exploring more complex chemical phenomena. By mastering the differences between these fundamental types of changes, a deeper understanding of topics such as reaction stoichiometry, energy transfer in chemical processes, and the properties of various states of matter becomes attainable.
Tips for Investigating Material Transformations in the Laboratory
Careful observation and meticulous record-keeping are crucial for successful experimentation in differentiating between physical and chemical changes. The following tips provide guidance for conducting effective investigations.
Tip 1: Prioritize Safety. Always wear appropriate personal protective equipment, including safety goggles and gloves. Ensure proper ventilation and follow all laboratory safety guidelines.
Tip 2: Document Initial Observations. Thoroughly record the initial properties of materials before any manipulations. Note characteristics such as color, texture, state (solid, liquid, gas), and any other relevant features.
Tip 3: Control the Variables. Whenever possible, change only one variable at a time to isolate its effects and draw accurate conclusions about the type of change occurring.
Tip 4: Monitor for Evidence of Chemical Change. Pay close attention to indicators of chemical reactions, including the production of gas (bubbles), formation of a precipitate (solid), color change, temperature change (release or absorption of heat), or the emission of light.
Tip 5: Document Thoroughly. Maintain detailed records of all procedures, observations, and measurements throughout the experiment. Use clear and concise language, and include units for all measurements.
Tip 6: Consider Energy Transfer. Observe whether heat is released or absorbed during the process, as this can be a key indicator of chemical change.
Tip 7: Dispose of Materials Properly. Follow proper waste disposal procedures as instructed by the laboratory supervisor. Different materials require different disposal methods.
Adhering to these practices will enhance experimental accuracy, promote safety, and contribute to a deeper understanding of the nature of physical and chemical processes.
By carefully observing and documenting the transformations of matter, a stronger foundation for more advanced chemical explorations can be established.
1. Substance Alteration
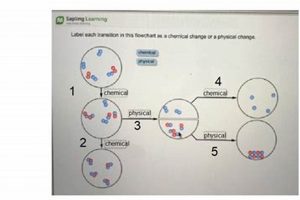
Substance alteration lies at the heart of distinguishing between chemical and physical changes within a laboratory setting. A chemical change fundamentally alters the substance at the molecular level, resulting in the formation of new compounds with different properties. This contrasts sharply with physical changes, where the substance retains its original chemical composition despite alterations to its form or state. For instance, the combustion of methane gas in the presence of oxygen yields carbon dioxide and water, entirely new substances. This exemplifies a chemical alteration. Conversely, the melting of ice simply transforms water from a solid to a liquid state without any change in its chemical identity, demonstrating a physical change.
The careful observation and analysis of substance alteration are crucial components of a “chemical vs physical change lab.” By meticulously documenting alterations in properties like color, odor, and the production of gas or precipitates, investigators can determine whether a new substance has formed. Consider the reaction of iron with sulfur. When heated, these two elements combine chemically to produce iron sulfide, a compound with distinct properties from its constituent elements. This formation of a new substance is a hallmark of chemical change. In contrast, dissolving sugar in water leads to no new substances; the sugar molecules are simply dispersed within the water, readily recoverable through evaporation. This preservation of original substances defines a physical change.
Understanding substance alteration has profound practical significance. It allows for the prediction and control of chemical reactions, crucial in fields like materials science, pharmaceuticals, and environmental science. Recognizing that burning fossil fuels generates new substances (carbon dioxide, water, and pollutants) highlights the environmental impact of these chemical changes. Conversely, understanding that physical processes like the evaporation of water do not alter its fundamental composition is crucial for water purification and resource management. Mastering this concept provides a foundational understanding of how matter interacts and transforms, underpinning advancements across diverse scientific disciplines.
2. Property Changes
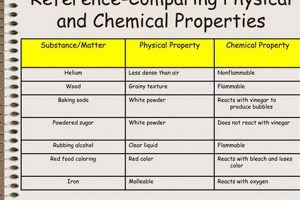
Observing property changes forms a cornerstone of investigations differentiating chemical and physical transformations within a laboratory setting. Alterations in properties serve as crucial indicators, providing evidence to classify the nature of the change. While both physical and chemical changes exhibit property changes, the types of alterations differ significantly. Physical changes typically involve modifications in state, form, or appearance, such as melting point, boiling point, density, or color intensity. For instance, the transition of water from ice to liquid water involves a change in state, but the substance remains chemically unchanged. Conversely, chemical changes manifest as alterations in chemical composition, resulting in new substances with distinct properties. The reaction between vinegar and baking soda, producing carbon dioxide gas, exemplifies this, with the evolution of gas signifying a new substance formation. This difference is fundamental to the “chemical vs physical change lab.”
Investigating property changes requires careful observation and measurement. Quantitative measurements, like temperature changes during a reaction, can signal the occurrence of a chemical change. Qualitative observations, such as a color shift during the rusting of iron, also provide valuable insights. The evolution of a gas, often observed as bubbling, signifies the production of a new substance, a key indicator of chemical change. Formation of a precipitate, an insoluble solid formed from a liquid reaction, also strongly suggests a chemical transformation. Consider the reaction of silver nitrate with sodium chloride, producing a white precipitate of silver chloride. The appearance of this new solid, distinct from the reactants, clearly marks a chemical change. Conversely, dissolving salt in water, while resulting in a homogenous mixture, does not generate new substances and is thus classified as a physical change.
The practical implications of understanding property changes in the context of chemical and physical transformations are far-reaching. In material science, recognizing the properties associated with specific changes allows for the development of materials with tailored characteristics. Within the realm of environmental science, understanding these changes provides insights into processes like the weathering of rocks or the formation of pollutants. Distinguishing between the property changes indicative of chemical and physical processes provides a foundational framework for comprehending the behavior of matter in diverse contexts. This foundational knowledge equips individuals with the analytical tools to interpret observations accurately and predict the outcomes of various processes, contributing to advancements in numerous scientific and technological domains.
3. Reversibility
Reversibility serves as a critical distinguishing feature in the classification of material transformations within a “chemical vs physical change lab” context. Physical changes are typically easily reversible, meaning the original substance can be recovered through straightforward processes. Melting ice, for instance, is readily reversed by cooling the liquid water back to its solid state. Similarly, dissolving salt in water can be reversed by evaporating the water, leaving the salt behind. This ease of reversal stems from the fact that the fundamental chemical identity of the substance remains unaltered during a physical change. In contrast, chemical changes are generally irreversible, or require complex procedures to revert. Combustion, a prime example, produces new substances like carbon dioxide and water from the original fuel and oxygen. Reconverting these products back into the original reactants requires multiple intricate chemical reactions.
The concept of reversibility has significant implications for experimental design and interpretation within the lab. When assessing whether a change is chemical or physical, attempting to reverse the process provides valuable evidence. If the original substance can be easily recovered, this strongly suggests a physical change. However, failure to revert the change easily does not automatically imply a chemical change. Some physical changes, like dissolving certain gases in liquids, can be challenging to reverse without specialized equipment. Conversely, some seemingly irreversible chemical changes can be reversed under specific conditions, like the electrolysis of water to produce hydrogen and oxygen gas, which can then be recombined through combustion to form water again. Therefore, reversibility should be considered alongside other indicators like the formation of new substances, changes in properties, and energy transfer.
Understanding reversibility in the context of material transformations has practical implications across various scientific disciplines. In materials science, recognizing the reversibility of certain phase transitions, such as the hardening and softening of metals through heating and cooling, allows for the manipulation of material properties for specific applications. Environmental science benefits from the understanding that some chemical changes, like the release of certain pollutants into the atmosphere, are difficult or impossible to reverse, highlighting the importance of preventative measures. Ultimately, reversibility plays a crucial role in interpreting experimental observations and developing a robust understanding of the nature of physical and chemical processes.
4. Energy Transfer
Energy transfer plays a crucial role in understanding the distinction between chemical and physical changes in a laboratory setting. All changes in matter involve some form of energy transfer, but the nature and magnitude of this transfer differ significantly between chemical and physical processes. Examining energy changes provides valuable insights into the underlying mechanisms driving these transformations.
- Exothermic and Endothermic Processes
Chemical changes often involve substantial energy changes, typically manifested as heat release (exothermic reactions) or heat absorption (endothermic reactions). Combustion reactions, like burning wood, release significant heat, while reactions like dissolving ammonium nitrate in water absorb heat from the surroundings. In contrast, physical changes generally involve smaller energy transfers. Melting ice, for example, requires energy input, while freezing water releases energy, but these energy changes are less dramatic than those seen in chemical reactions. Observing and quantifying these energy transfers in a laboratory setting provides valuable data for differentiating between chemical and physical changes.
- Heat of Reaction and Phase Changes
The energy transfer associated with a chemical change is often referred to as the heat of reaction. This can be measured using calorimetry techniques, providing quantitative data that can be used to characterize the reaction. In physical changes, energy transfer is primarily associated with phase transitions, like melting or boiling. The energy required to melt a substance is its heat of fusion, while the energy needed to vaporize a substance is its heat of vaporization. These values are characteristic properties of substances and can be used to identify them. Laboratory experiments can demonstrate these energy transfers by measuring temperature changes during phase transitions, providing further evidence for classifying the change as physical.
- Energy Conservation
Regardless of whether a change is chemical or physical, the principle of energy conservation always applies. Energy cannot be created or destroyed, only transferred or converted from one form to another. In chemical reactions, the energy stored in chemical bonds is either released or absorbed, typically in the form of heat. In physical changes, energy transfer is associated with changes in intermolecular forces. For example, when ice melts, energy is absorbed to overcome the intermolecular forces holding water molecules in a rigid crystalline structure. Understanding energy conservation helps interpret observations in a “chemical vs physical change lab,” ensuring that the total energy input and output are accounted for.
- Activation Energy
While not directly measurable in typical “chemical vs physical change labs,” the concept of activation energy is relevant for understanding chemical transformations. Activation energy is the minimum energy required to initiate a chemical reaction. Physical changes do not have an activation energy requirement. The presence or absence of an activation energy barrier contributes to differentiating between chemical and physical changes at a theoretical level, even if not directly observed in basic experimental setups.
By carefully considering the type, magnitude, and direction of energy transfer, a deeper understanding of the nature of the changes occurring within a laboratory setting can be achieved. Observations of energy transfer, combined with other indicators like substance alteration and reversibility, provide a comprehensive framework for classifying and understanding material transformations.
5. Experimental Observation
Experimental observation forms the cornerstone of any investigation into the nature of matter transformations, particularly within the context of a “chemical vs physical change lab.” Accurate and detailed observations are essential for distinguishing between chemical and physical changes, providing the empirical evidence upon which conclusions are drawn. This involves careful monitoring of the system under investigation, noting any changes in properties, and meticulously recording all relevant data. The reliability of experimental observations directly impacts the validity of conclusions regarding the type of change occurring.
- Qualitative Observations
Qualitative observations, such as changes in color, odor, or the formation of a precipitate, provide valuable initial indicators in a “chemical vs physical change lab.” For example, observing a color change upon mixing two solutions may suggest a chemical reaction has occurred. Similarly, the formation of a precipitate indicates the creation of a new, insoluble substance, another hallmark of chemical change. While qualitative observations provide crucial initial insights, they are often subjective and require further investigation through quantitative measurements for confirmation.
- Quantitative Measurements
Quantitative measurements provide objective data crucial for analyzing changes in a “chemical vs physical change lab.” Measurements of temperature changes during a reaction, volume of gas produced, or mass of precipitate formed offer precise evidence for characterizing the transformation. For instance, measuring a temperature increase during a reaction suggests an exothermic process, often associated with chemical changes. Quantitative measurements allow for more rigorous analysis and comparison between different experiments, strengthening conclusions about the nature of the change.
- Systematic Documentation
Systematic documentation is critical for ensuring the reliability and reproducibility of experimental observations in a “chemical vs physical change lab.” Detailed records of experimental procedures, initial conditions, observed changes, and quantitative measurements allow for thorough analysis and interpretation. This documentation also enables others to replicate the experiment, validating the observations and conclusions. Maintaining accurate records is essential for drawing valid conclusions and contributing to the broader scientific understanding of chemical and physical changes.
- Data Interpretation and Analysis
Experimental observations, both qualitative and quantitative, must be carefully interpreted and analyzed to draw meaningful conclusions in a “chemical vs physical change lab.” This involves comparing the observed changes with known indicators of chemical and physical changes. For instance, observing a color change alone may not be conclusive evidence of a chemical change, as some physical changes, like dilution, can also result in color alterations. The analysis should consider multiple factors, including reversibility, energy transfer, and substance alteration, to arrive at a well-supported conclusion about the type of change observed. Careful data interpretation is essential for connecting experimental observations to underlying scientific principles.
The synergy of these facets of experimental observation provides a powerful framework for understanding material transformations. By combining careful qualitative observations with precise quantitative measurements, systematically documenting the process, and rigorously interpreting the data, a comprehensive understanding of the distinction between chemical and physical changes can be achieved within a “chemical vs physical change lab.” This approach strengthens experimental conclusions and contributes to the broader scientific knowledge base concerning matter and its interactions.
6. Safety Precautions
Safety precautions are paramount in any laboratory setting, particularly when investigating chemical and physical changes. Experiments often involve potentially hazardous substances and procedures, necessitating stringent safety protocols to minimize risks. A “chemical vs physical change lab” typically involves manipulating substances and observing reactions, some of which may produce toxic fumes, flammable gases, or corrosive materials. For instance, burning magnesium, a common demonstration of chemical change, produces intense heat and bright light, requiring eye protection and careful handling to prevent burns. Similarly, experiments involving acids and bases necessitate the use of gloves and appropriate ventilation to minimize exposure to corrosive substances. Ignoring safety precautions can lead to accidents, injuries, and exposure to hazardous materials. Therefore, understanding and adhering to established safety protocols is essential for ensuring a safe and productive learning environment.
Several key safety precautions are universally applicable within a “chemical vs physical change lab” setting. Eye protection, typically in the form of safety goggles, is crucial to prevent eye damage from chemical splashes, projectiles, or intense light. Gloves provide a barrier against skin contact with corrosive or irritating substances. Proper ventilation, achieved through fume hoods or open windows, minimizes inhalation of potentially harmful gases. Additionally, adhering to proper waste disposal procedures is essential to prevent environmental contamination and further hazards. For example, certain chemical waste must be neutralized or collected separately, while broken glassware requires specific disposal methods. Furthermore, understanding the properties of the substances involved is crucial. Knowing the flammability, reactivity, and toxicity of materials informs safe handling practices and appropriate responses to potential incidents. Material Safety Data Sheets (MSDS) provide comprehensive information about the hazards associated with specific substances and should be consulted before handling any unfamiliar material. A well-maintained and organized laboratory environment, with designated areas for specific procedures and clearly labeled chemicals, also contributes significantly to overall safety.
A comprehensive understanding of safety procedures and their rigorous application are integral components of successful scientific inquiry within a “chemical vs physical change lab.” Proper safety practices not only protect individuals from immediate harm but also cultivate a culture of responsibility and respect for the potential hazards inherent in scientific exploration. Integrating safety considerations into experimental design and execution ensures a secure environment conducive to learning and discovery. Challenges may arise in ensuring consistent adherence to safety protocols, especially among inexperienced individuals. Addressing these challenges requires clear communication of safety guidelines, thorough training, and regular reinforcement of best practices. Prioritizing safety fosters a productive and enriching laboratory experience, enabling deeper engagement with the scientific principles being investigated.
7. Scientific Methodology
Scientific methodology provides the essential framework for rigorous investigation within a “chemical vs physical change lab.” Its systematic approach ensures reliable results and fosters a deeper understanding of the underlying principles governing matter transformations. Applying scientific methodology allows for objective analysis of experimental observations, contributing to the accurate classification of changes as either chemical or physical. This structured approach is crucial for distinguishing between superficial alterations and fundamental changes in substance composition.
- Hypothesis Formulation
A “chemical vs physical change lab” begins with formulating a testable hypothesis regarding the nature of the change expected to occur. For example, one might hypothesize that mixing two specific solutions will result in a chemical change due to the formation of a precipitate. This hypothesis guides the experimental design and provides a focus for observations and data collection. Clearly defined hypotheses are essential for drawing meaningful conclusions from experimental results.
- Experimental Design
Careful experimental design is crucial for obtaining reliable data. This involves controlling variables, ensuring accurate measurements, and selecting appropriate procedures. For instance, in an experiment investigating the effect of temperature on reaction rate, maintaining a constant temperature for each trial is essential. Precise measurements of mass, volume, and temperature are crucial for quantitative analysis. A well-designed experiment minimizes extraneous factors that could influence the outcome, isolating the effects of the variable being studied and allowing for clear interpretation of results within a “chemical vs physical change lab” context.
- Data Collection and Analysis
Systematic data collection and analysis are fundamental to drawing valid conclusions. Accurate recording of observations, both qualitative and quantitative, provides the raw material for analysis. For example, noting a color change, measuring the temperature change during a reaction, or calculating the mass of a precipitate formed contributes to the dataset. Analyzing this data through calculations, graphing, or statistical analysis reveals patterns and trends, providing evidence to support or refute the initial hypothesis. Rigorous data analysis ensures that conclusions drawn are based on empirical evidence, a cornerstone of scientific methodology.
- Interpretation and Conclusion
Interpretation of the analyzed data involves comparing the experimental findings with the initial hypothesis and established scientific principles. Determining whether the observed changes align with the predicted outcomes is crucial. If the results contradict the hypothesis, re-evaluation of the hypothesis or experimental design may be necessary. Conclusions should be based on the totality of evidence gathered and should address the initial research question. For example, if experimental data confirms the formation of a new substance with distinct properties, the conclusion would support the classification of the change as chemical. Drawing valid conclusions based on evidence and logical reasoning exemplifies the application of scientific methodology in a “chemical vs physical change lab.”
The rigorous application of scientific methodology in a “chemical vs physical change lab” ensures that experimental findings are reliable and contribute to a deeper understanding of the nature of matter. This structured approach empowers individuals to differentiate between chemical and physical changes based on empirical evidence and sound reasoning, facilitating a more profound comprehension of the transformations occurring at the molecular level. The emphasis on hypothesis testing, controlled experimentation, and data-driven analysis provides a robust framework for exploring the fascinating world of chemical and physical phenomena.
Frequently Asked Questions
Addressing common inquiries regarding the differentiation between chemical and physical changes in a laboratory setting clarifies conceptual understanding and promotes effective experimental design.
Question 1: How does the formation of a precipitate indicate a chemical change?
Precipitate formation signifies the creation of a new, insoluble substance from a reaction between soluble reactants. This distinct product, with properties different from the original reactants, marks a chemical transformation.
Question 2: Is a change in color always indicative of a chemical change?
While color change often accompanies chemical reactions, it’s not exclusively indicative of them. Physical changes, such as dilution or changes in physical state, can also alter color. Consider other factors like new substance formation for accurate classification.
Question 3: If no visible change occurs, can one assume no reaction took place?
Absence of visible change doesn’t preclude a reaction. Some chemical reactions produce no immediately observable macroscopic changes. Careful monitoring of other indicators, like temperature change or gas production, is essential.
Question 4: How does the reversibility of a process help determine its nature?
Physical changes are generally easily reversible, meaning the original substance can be recovered through simple processes. Chemical changes, however, are typically irreversible, or require complex procedures to revert due to the formation of new substances.
Question 5: Why is understanding energy transfer crucial in distinguishing between these changes?
Energy transfer accompanies all changes, but the magnitude differs. Chemical changes often involve substantial energy changes, whereas physical changes involve relatively smaller energy transfers, primarily associated with changes in intermolecular forces or phase transitions.
Question 6: What’s the role of scientific methodology in these investigations?
Scientific methodology ensures rigorous and reliable experimentation. Hypothesis formulation, controlled experiments, systematic data collection, and logical analysis are crucial for drawing valid conclusions about the nature of the observed transformations.
Accurately discerning between chemical and physical changes hinges on careful observation, meticulous experimentation, and a thorough understanding of underlying chemical principles. These frequently asked questions provide a framework for approaching experimental investigations and interpreting observed phenomena.
This understanding lays the groundwork for further exploration of chemical reactions, stoichiometry, and the properties of matter.
Conclusion
Investigations centered on differentiating between chemical and physical changes provide fundamental insights into the nature of matter. Distinguishing between transformations that alter substance composition (chemical changes) and those that merely affect physical properties (physical changes) is crucial for understanding material behavior. Key differentiators explored include substance alteration, property changes, reversibility, and energy transfer. Meticulous experimental observation, incorporating both qualitative and quantitative data, is essential for accurate classification. Furthermore, adherence to established safety protocols is paramount given the potential hazards associated with laboratory work.
A robust understanding of the distinctions between chemical and physical changes empowers informed decision-making across scientific disciplines, from material science and chemical engineering to environmental science and medicine. Continued exploration and refinement of experimental techniques will further enhance comprehension of these fundamental processes, driving advancements in research and technology. The ability to manipulate and predict material transformations based on these principles holds significant promise for future innovations.