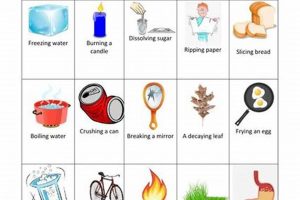
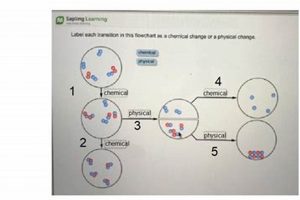
Experiments conducted in a controlled environment to observe transformations in matter can be categorized into two primary types: those involving alterations in composition and those involving alterations in form or state. Melting an ice cube, for instance, exemplifies a change in state, while burning wood represents a change in composition. Such investigations commonly involve observations of properties like color change, gas production, temperature variation, and precipitate formation.
Investigating these transformations is fundamental to understanding the nature of matter and its interactions. These core concepts form the basis for advancements across scientific disciplines, from materials science and chemical engineering to medicine and environmental science. Historical precedents, such as alchemists’ attempts to transmute base metals into gold, though ultimately unsuccessful, contributed to the development of early experimental techniques and laid the groundwork for modern chemistry.
The exploration of these fundamental principles often includes topics such as phase transitions, reaction stoichiometry, and energy transfer. A deeper understanding of these concepts offers valuable insights into the world around us, enabling innovations in fields like renewable energy, drug development, and materials engineering.
Tips for Effective Experimentation
Careful preparation and execution are essential for obtaining reliable and meaningful results when investigating transformations in matter.
Tip 1: Accurate Observation: Meticulous recording of all observed changes, including qualitative aspects like color and texture, and quantitative data such as temperature and mass, is crucial for accurate analysis.
Tip 2: Proper Safety Procedures: Adherence to established safety protocols, including wearing appropriate personal protective equipment (e.g., goggles, gloves) and handling chemicals with care, is paramount.
Tip 3: Controlled Environments: Conducting experiments in controlled environments minimizes external influences and ensures the reliability of observations. Maintaining consistent temperature and pressure, for example, is often critical.
Tip 4: Precise Measurements: Using calibrated instruments and employing proper measurement techniques ensures the accuracy of quantitative data collection. This includes reading measurements at eye level to avoid parallax errors.
Tip 5: Replication: Repeating experiments multiple times confirms the consistency of results and increases confidence in the validity of conclusions.
Tip 6: Data Analysis: Careful analysis of collected data, including calculations and graphical representations, allows for the identification of trends and the drawing of meaningful conclusions.
Tip 7: Experimental Design: A well-designed experiment includes a clear hypothesis, carefully chosen variables, and appropriate controls. This structured approach facilitates accurate interpretation of results.
Adhering to these practices ensures experimental integrity and allows for the reliable investigation of the properties of matter and its transformations. These foundational principles support scientific advancement across diverse disciplines.
By understanding and applying these tips, one can effectively explore the fascinating world of matter and its interactions.
1. Observation
Observation forms the cornerstone of understanding transformations in matter within a controlled laboratory setting. It provides the raw data upon which interpretations and conclusions are built, serving as the foundation for distinguishing between alterations in substance composition and alterations in physical state.
- Qualitative Observations
Qualitative observations describe characteristics perceived through the senses, without numerical measurements. Examples include noting a color change during a reaction (e.g., clear solutions turning blue) or observing the formation of a precipitate. These observations provide crucial descriptive data in experimental investigations.
- Quantitative Observations
Quantitative observations involve numerical measurements. These include measuring temperature changes during a reaction, determining the mass of a substance, or recording the volume of gas produced. Precise quantitative measurements are essential for accurate data analysis and drawing meaningful conclusions.
- Systematic Observation
Systematic observation involves a structured approach to data collection, ensuring all relevant details are captured. Using a pre-designed data table to record observations at regular intervals exemplifies systematic observation. This methodical approach minimizes bias and enhances the reliability of findings.
- Interpreting Observations
Observations, whether qualitative or quantitative, require careful interpretation to derive meaning. A temperature increase during a reaction, for instance, could indicate an exothermic process. Linking observations to underlying scientific principles allows for a deeper understanding of the observed phenomena.
The interplay of these observational facets contributes significantly to the success of investigations in the study of matter. By systematically collecting and interpreting both qualitative and quantitative observations, researchers gain insights into the fundamental principles governing transformations in matter, facilitating advancements across scientific disciplines.
2. Measurement
Measurement plays a critical role in the investigation of transformations in matter within a controlled laboratory setting. Accurate and precise measurements provide the quantitative data necessary to understand the nature and extent of these changes. Whether examining alterations in composition or physical state, quantifiable data allows for objective analysis and the development of evidence-based conclusions. The relationship between measurement and the study of these transformations is one of fundamental interdependence.
Consider the example of determining the mass of a substance before and after a chemical reaction. Precise measurement of mass allows for the calculation of mass changes, which can provide insights into the stoichiometry of the reaction and the conservation of mass. Similarly, measuring temperature changes during a reaction offers valuable information about the energy transfer associated with the transformation. Whether the reaction is exothermic or endothermic can be determined by careful temperature monitoring. Measuring the volume of gas evolved during a reaction provides another quantifiable metric for understanding the extent and nature of the change. These examples highlight the practical significance of accurate measurement in understanding transformations in matter. Without precise measurements, interpretations of observations would remain qualitative and less conclusive.
The use of calibrated instruments and appropriate measurement techniques is crucial for obtaining reliable data. Systematic recording of measurements, coupled with proper error analysis, ensures data integrity and facilitates meaningful comparisons. Challenges in measurement, such as limitations in instrument precision or potential sources of experimental error, must be acknowledged and addressed to ensure the validity of findings. Ultimately, precise measurement provides the quantitative foundation upon which understanding of chemical and physical changes is built, enabling advancements in scientific knowledge and its applications.
3. Experimentation
Experimentation forms the core of investigations within a chemical physical changes lab, providing a structured framework for exploring transformations in matter. Through carefully designed experiments, researchers can systematically manipulate variables, observe outcomes, and gather data to understand the nature of these changes, whether they involve alterations in composition or simply changes in physical state.
- Hypothesis Testing
A central element of experimentation is hypothesis testing. A hypothesis, a proposed explanation for an observed phenomenon, is tested through controlled experiments. In the context of a chemical physical changes lab, a hypothesis might predict the outcome of mixing two substances or the effect of temperature on a reaction rate. Experimental results either support or refute the hypothesis, leading to a deeper understanding of the underlying principles governing the transformation.
- Controlled Variables
Manipulating and controlling variables is crucial for valid experimental results. Independent variables are deliberately changed, while dependent variables are measured to observe the effects of the manipulation. Controlling extraneous variables, such as temperature or pressure, ensures that observed changes are attributable solely to the independent variable. For example, when investigating the effect of temperature on reaction rate, temperature would be the independent variable, the reaction rate the dependent variable, and factors like reactant concentrations would be carefully controlled.
- Data Collection and Analysis
Systematic data collection is essential during experimentation. This includes both qualitative observations, such as color changes, and quantitative measurements, like temperature readings or mass changes. Collected data is then analyzed to identify trends, patterns, and relationships between variables. Graphical representation of data often aids in visualizing these relationships and drawing meaningful conclusions. Data analysis provides the evidence base for interpreting experimental outcomes.
- Experimental Design
Effective experimentation relies on a well-considered experimental design. This includes defining a clear research question, formulating a testable hypothesis, selecting appropriate experimental procedures, and identifying potential sources of error. A robust experimental design ensures the reliability and validity of the results, enabling researchers to draw accurate conclusions about the nature and characteristics of the transformations under investigation. Variations in experimental design can be explored to optimize data collection and address specific research questions.
These facets of experimentation, working in concert, provide a powerful framework for understanding transformations in matter within a chemical physical changes lab. By systematically manipulating variables, collecting data, and analyzing results, researchers gain insights into the underlying chemical and physical principles that govern these changes, contributing to advancements in scientific knowledge and its applications.
4. Analysis
Analysis forms an integral component of investigations conducted within a chemical physical changes lab. It provides the framework for interpreting experimental data and drawing meaningful conclusions regarding transformations in matter. Whether examining alterations in composition or changes in physical state, analysis bridges the gap between raw data and scientific understanding. Analysis within this context encompasses several key aspects: evaluating observed changes, interpreting quantitative measurements, and drawing conclusions based on the totality of evidence gathered. For instance, a change in color observed during a reaction, coupled with a measured temperature increase, might suggest a specific chemical transformation. Analysis involves integrating these distinct pieces of information to form a coherent interpretation. This process often requires applying theoretical knowledge, such as understanding reaction mechanisms or phase transitions, to explain the observed phenomena. Analysis might involve comparing experimental results to established scientific principles to confirm or refine existing theories. Discrepancies between observed and expected outcomes can stimulate further investigation and lead to new discoveries.
Quantitative data analysis frequently involves statistical methods to determine the reliability and significance of results. Calculating error margins, evaluating standard deviations, and performing statistical tests provide a quantitative basis for assessing the validity of conclusions drawn from experimental data. Furthermore, graphical representation of data, such as plotting reaction rates against temperature, can reveal trends and relationships that might not be readily apparent from raw numerical data. Such visualizations facilitate a deeper understanding of the processes under investigation. Analysis also encompasses identifying potential sources of error and assessing their impact on experimental outcomes. This critical evaluation of data quality strengthens the conclusions drawn and informs future experimental design.
Effective analysis within a chemical physical changes lab relies on both scientific rigor and a nuanced understanding of the specific system under investigation. It demands careful consideration of all available evidence, both qualitative and quantitative, and a willingness to revise interpretations in light of new information. By meticulously analyzing experimental data, researchers can uncover the fundamental principles governing transformations in matter, ultimately contributing to advances across scientific disciplines. The ability to analyze and interpret data accurately is thus an essential skill for anyone engaged in the study of chemical and physical changes.
5. Safety
Safety is paramount in environments where investigations into the transformations of matter occur. These investigations, involving manipulations of substances and observation of their interactions, necessitate stringent safety protocols to mitigate potential hazards and ensure the well-being of all involved. Understanding and adhering to these procedures is not merely a recommendation, but a fundamental requirement for responsible scientific practice.
- Personal Protective Equipment (PPE)
Proper use of PPE is the first line of defense against potential hazards. Eye protection (goggles), hand protection (gloves), and protective clothing (lab coats) are essential to prevent exposure to chemicals, heat, and other potential hazards. For instance, goggles protect the eyes from splashes of corrosive substances or flying debris, while gloves prevent skin contact with irritants or toxins. Choosing the appropriate PPE for the specific experiment is crucial, considering factors like chemical compatibility and the potential for thermal or mechanical hazards.
- Chemical Handling
Safe handling of chemicals requires meticulous attention to detail. This includes proper storage, dispensing, and disposal procedures. Chemicals should be stored in clearly labeled containers, away from incompatible substances. When dispensing chemicals, appropriate techniques should be used to avoid spills or contamination. Waste disposal must adhere to strict guidelines, ensuring that chemicals are neutralized or disposed of in designated waste streams. For example, strong acids and bases should never be mixed directly, and volatile chemicals should be handled under a fume hood to minimize inhalation risks. Understanding chemical properties and potential hazards is crucial for safe handling.
- Emergency Procedures
Knowledge of emergency procedures is essential for responding effectively to unforeseen events. This includes knowing the location and proper use of safety equipment such as fire extinguishers, eyewash stations, and safety showers. Evacuation routes and assembly points should be clearly understood. In the event of a chemical spill, appropriate cleanup procedures should be followed, using spill kits and neutralizers if necessary. Prompt and appropriate action in emergencies can significantly mitigate potential harm. Regular safety drills reinforce these procedures and ensure preparedness.
- Risk Assessment
Prior to conducting any experiment, a thorough risk assessment should be performed. This involves identifying potential hazards, evaluating the likelihood and severity of those hazards, and implementing control measures to minimize risks. For example, when working with flammable materials, ensuring adequate ventilation and eliminating ignition sources are essential control measures. The risk assessment should be documented and communicated to all individuals involved in the experiment. This proactive approach to safety management promotes a culture of safety and minimizes the potential for accidents.
These facets of safety, working in concert, create a secure environment for investigating transformations in matter within a chemical physical changes lab. Prioritizing safety not only protects individuals but also ensures the integrity of the scientific process, allowing for reliable and meaningful results. Negligence in any of these areas can compromise the safety of individuals and the validity of experimental findings. A commitment to safety is therefore an indispensable element of responsible scientific practice.
6. Interpretation
Interpretation within a chemical physical changes lab is the process of assigning meaning to observations and experimental data. It is the bridge between empirical evidence and scientific understanding, transforming raw data into explanations of the observed transformations in matter. Whether analyzing a change in composition or a shift in physical state, accurate interpretation is crucial for drawing valid conclusions and advancing scientific knowledge. Interpretation is not merely a passive reading of results; it requires critical thinking, the application of theoretical frameworks, and the integration of multiple lines of evidence.
- Connecting Observations to Theory
Interpretation involves linking observed phenomena to established scientific theories. For example, if a reaction produces a gas and a temperature increase, the interpretation might involve connecting these observations to the theory of exothermic reactions and gas evolution. This connection requires understanding the underlying chemical principles and applying them to the specific experimental context. The observed gas could be identified using qualitative tests, further strengthening the interpretation. Similarly, if a substance transitions from solid to liquid upon heating, the interpretation would link this observation to the theory of phase transitions and melting points. This connection provides a theoretical framework for understanding the observed physical change.
- Explaining Data Trends
Experimental data often reveals trends and patterns that require careful interpretation. For instance, if the rate of a reaction increases with increasing temperature, the interpretation might invoke the collision theory, explaining the increased rate in terms of more frequent and energetic collisions between reactant molecules. Graphical analysis, such as plotting reaction rate against temperature, can aid in visualizing these trends and formulating a coherent interpretation. Similarly, if the solubility of a substance increases with temperature, the interpretation might relate this trend to the thermodynamics of dissolution. This connection provides a deeper understanding of the factors influencing solubility.
- Evaluating Sources of Error
Experimental data is rarely perfect. Interpretation must consider potential sources of error and their impact on the results. If a measured mass change is smaller than expected, the interpretation should consider potential sources of error such as incomplete reaction or loss of product during transfer. This critical evaluation of data quality strengthens the validity of the interpretation. Similarly, if the observed melting point of a substance is lower than the literature value, the interpretation should consider potential sources of error such as impurities in the sample or inaccuracies in temperature measurement.
- Formulating Conclusions
The ultimate goal of interpretation is to formulate conclusions about the transformations under investigation. Based on the totality of evidence, including observations, quantitative data, and theoretical considerations, the interpretation culminates in a reasoned explanation of the observed phenomena. For example, the conclusion of an experiment might be that a specific chemical reaction has occurred, producing a particular product, or that a substance has undergone a phase transition from solid to liquid at a specific temperature. These conclusions contribute to the body of scientific knowledge and inform further investigations.
In the context of a chemical physical changes lab, interpretation is not merely a post-experiment activity; it is an ongoing process that informs experimental design, data collection, and analysis. The ability to accurately interpret observations and experimental data is essential for understanding the fundamental principles governing transformations in matter and advancing scientific knowledge. Effective interpretation relies on a combination of critical thinking, theoretical knowledge, and practical experience. It is a dynamic process, continually refined as new evidence emerges and scientific understanding evolves.
Frequently Asked Questions
This section addresses common inquiries regarding the investigation of transformations in matter within a controlled laboratory setting.
Question 1: What key indicators distinguish a chemical change from a physical change?
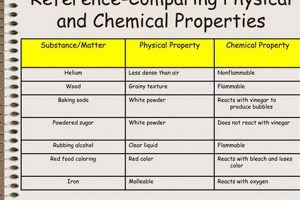
Chemical changes involve alterations in the composition of matter, resulting in the formation of new substances with different properties. Indicators include gas production, color change, temperature change, precipitate formation, and light emission. Physical changes, conversely, involve alterations in the form or state of matter without changes in composition, such as melting, boiling, or dissolving.
Question 2: How does controlling variables enhance the reliability of experimental results?
Controlling variables isolates the impact of the independent variable on the dependent variable, minimizing the influence of extraneous factors. This ensures that observed changes are attributable to the manipulated variable, enhancing the reliability and validity of conclusions.
Question 3: Why is precise measurement essential in these investigations?
Precise measurement provides quantitative data crucial for understanding the extent and nature of transformations. Accurate measurements enable calculations, comparisons, and trend analysis, facilitating a more rigorous understanding of the processes under investigation.
Question 4: What is the role of a control group in experimental design?
A control group provides a baseline for comparison, allowing researchers to isolate the effect of the independent variable. The control group is identical to the experimental group in all aspects except for the manipulated variable, enabling a clear assessment of its impact.
Question 5: How does a risk assessment contribute to laboratory safety?
A risk assessment systematically identifies potential hazards and evaluates their likelihood and severity. This information informs the development of control measures to minimize risks, ensuring a safe environment for conducting experiments. It is a crucial proactive safety measure.
Question 6: Why is replication important in scientific investigations?
Replication of experiments ensures that results are consistent and not due to chance or experimental error. Repeated trials increase confidence in the validity of findings and strengthen the reliability of conclusions.
Understanding these fundamental principles is essential for conducting meaningful investigations into the nature of matter and its transformations. This knowledge provides a foundation for scientific discovery and advancement across various disciplines.
Further exploration of specific experimental techniques and analytical methods can deepen understanding and enhance practical skills in investigating transformations in matter.
Conclusion
Investigations conducted within controlled laboratory environments, focused on transformations in matter, provide fundamental insights into the nature of chemical and physical changes. Careful observation, precise measurement, and rigorous experimental design are crucial for distinguishing between alterations in composition (chemical changes) and alterations in form or state (physical changes). Analysis of experimental data, informed by established scientific principles, allows for the interpretation of observed phenomena and the formulation of valid conclusions. Prioritizing safety through risk assessment and adherence to established protocols is paramount for ensuring a secure and productive research environment. From understanding reaction mechanisms to characterizing phase transitions, the meticulous exploration of these transformations provides a cornerstone for advancements across diverse scientific disciplines.
Continued investigation into these fundamental processes offers a pathway to deeper understanding of the material world. Further research promises advancements in fields ranging from materials science and chemical engineering to medicine and environmental science. Rigorous exploration of these transformations remains essential for addressing critical challenges and driving technological innovation.