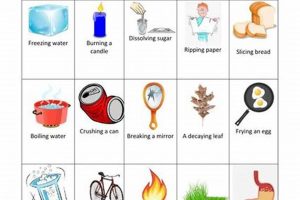
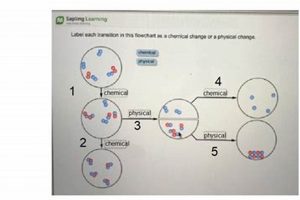
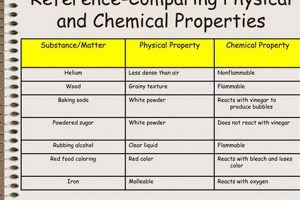
Investigations into transformations of matter are frequently undertaken in educational settings. These explorations typically involve designing and conducting experiments to observe and document alterations in substances, categorizing these alterations as either chemical, involving changes in composition, or physical, involving changes in form or state without altering composition. Examples include observing the rusting of iron (chemical) or the melting of ice (physical). These practical exercises often culminate in a structured presentation of findings.
Such studies are fundamental to building a strong foundation in scientific principles. They foster critical thinking skills through observation, data collection, analysis, and interpretation. Historically, understanding these transformations has been essential for advancements in numerous fields, from developing new materials to understanding biological processes. A grasp of these concepts empowers individuals to interact with the world around them with a deeper understanding of the underlying processes that govern it.
The following sections will delve further into distinguishing between these two types of transformations, explore common experimental designs, and discuss effective methods for data analysis and presentation.
Tips for Investigating Transformations of Matter
Successful investigations of matter transformations require careful planning and execution. The following tips offer guidance for conducting effective experiments and analyzing results.
Tip 1: Clearly Define Objectives. Before commencing any experiment, specific research questions should be formulated. This ensures focused data collection and analysis.
Tip 2: Control Variables Rigorously. Maintaining consistent experimental conditions, except for the independent variable, is crucial for accurate interpretation. This isolates the impact of the manipulated variable.
Tip 3: Document Observations Meticulously. Detailed records of all observations, including quantitative measurements and qualitative descriptions, are essential for drawing valid conclusions.
Tip 4: Ensure Proper Safety Precautions. Handling chemicals and equipment safely is paramount. Appropriate safety protocols should be followed throughout the investigation, including wearing appropriate personal protective equipment.
Tip 5: Employ Appropriate Analytical Techniques. Selecting the correct methods for data analysis is crucial for revealing meaningful insights. This may involve calculations, graphing, or statistical analysis.
Tip 6: Interpret Results Objectively. Conclusions should be drawn based solely on the evidence gathered, avoiding bias or preconceived notions. Limitations of the experiment should also be acknowledged.
Tip 7: Communicate Findings Effectively. Presenting findings clearly and concisely, using appropriate visual aids and terminology, ensures effective communication of the scientific process and results.
Adhering to these guidelines facilitates robust investigations, leading to a deeper understanding of the nature of matter and its transformations. This rigorous approach cultivates essential scientific skills applicable to a broad range of disciplines.
By understanding and applying these principles, investigations into the nature of matter can be conducted effectively, leading to a deeper understanding of scientific principles and the world around us.
1. Observation
Observation forms the cornerstone of any investigation into the transformations of matter. It provides the raw data upon which classifications of changes as physical or chemical are based. Careful observation involves noting initial states, documenting any alterations during the process, and recording final states. The quality and detail of observations directly influence the validity and reliability of subsequent analysis. For instance, observing the formation of a precipitate when two solutions are mixed suggests a chemical change, while observing a change in volume upon heating points to a physical change. Without meticulous observation, crucial details might be overlooked, leading to misinterpretations of the transformative processes. The acuity of observation skills directly impacts the accuracy of distinguishing between physical and chemical changes.
Furthermore, observation extends beyond simply noting changes; it encompasses precise measurement and detailed description. Quantitative data, such as temperature changes, mass differences, or volume alterations, provide crucial evidence for characterizing transformations. Qualitative data, such as color changes, odor formation, or gas evolution, offer additional clues. The combination of quantitative and qualitative observations provides a comprehensive understanding of the process. For example, observing a change in temperature during a reaction provides quantitative data regarding energy transfer, while observing a change in color offers qualitative insights into the formation of new substances. The application of both types of observation strengthens the analysis of the transformation.
In summary, effective observation serves as the essential foundation for understanding transformations of matter. It necessitates attention to detail, accurate recording, and the integration of both quantitative and qualitative data. Developing strong observation skills is crucial for successfully conducting investigations and drawing valid conclusions about the nature of physical and chemical changes. These skills, once honed, become invaluable tools applicable to a wide range of scientific endeavors.
2. Classification
Classification stands as a critical step in any project investigating transformations of matter. Following observation, the collected data must be analyzed to determine whether observed changes represent physical or chemical transformations. This classification hinges on understanding the underlying processes governing the observed changes. Physical changes involve alterations in form, state, or appearance without affecting the substance’s chemical composition. Chemical changes, conversely, result in the formation of new substances with different chemical properties. The ability to accurately classify observed changes is essential for drawing meaningful conclusions about the nature of the transformation. For example, dissolving sugar in water represents a physical change, as the sugar molecules remain intact, merely dispersed within the water. Burning wood, however, constitutes a chemical change, as the wood reacts with oxygen to produce ash, carbon dioxide, and water entirely new substances.
Effective classification requires a thorough understanding of the defining characteristics of each type of change. Indicators of chemical changes include the production of gas, formation of a precipitate, color change, odor change, and temperature change. These indicators often signal the breaking and forming of chemical bonds, leading to a new substance. Physical changes, on the other hand, might involve changes in shape, size, state of matter (solid, liquid, gas), or density. Boiling water, for example, is a physical change as the water molecules remain chemically identical in both liquid and gaseous states. Conversely, the rusting of iron, involving the reaction of iron with oxygen to form iron oxide, clearly represents a chemical change. Accurately categorizing changes based on these characteristics ensures a sound understanding of the underlying processes. Differentiating between these two types of transformations is crucial for subsequent analysis and interpretation of the data.
Proper classification lays the groundwork for subsequent stages of a project investigating matter transformations. It allows for a deeper analysis of the observed phenomena, connecting observable changes to underlying molecular processes. This understanding, in turn, facilitates the development of accurate explanations and predictions regarding the behavior of matter. Challenges in classification often arise when changes exhibit characteristics of both physical and chemical transformations. For instance, dissolving certain salts in water involves both a physical change (dissolution) and a chemical change (ionization). In such cases, careful analysis and consideration of multiple factors are necessary for accurate classification. Mastering the ability to classify transformations of matter is essential for progressing from observation to understanding, laying the foundation for further scientific inquiry.
3. Experimentation
Experimentation forms the core of any project exploring chemical and physical changes, providing a structured approach to investigate cause-and-effect relationships. Well-designed experiments allow for controlled manipulation of variables, enabling researchers to isolate the impact of specific factors on the transformation of matter. This controlled approach is essential for distinguishing between correlation and causation, establishing a definitive link between the manipulated variable and the observed outcome. For example, an experiment investigating the rusting of iron might involve exposing identical iron samples to varying levels of humidity while controlling other factors like temperature and oxygen concentration. This isolates the effect of humidity on the rusting process, providing direct evidence for its role in the reaction. The design of the experiment dictates the strength of the conclusions drawn.
A crucial aspect of experimentation lies in the careful selection and manipulation of independent, dependent, and control variables. The independent variable, the factor being manipulated, must be clearly defined and controlled. The dependent variable, the measured outcome, provides insights into the effects of the independent variable. Control variables, kept constant throughout the experiment, minimize extraneous influences ensuring that the observed changes are solely attributable to the manipulated independent variable. For instance, in an experiment investigating the effect of temperature on reaction rate, temperature represents the independent variable, reaction rate the dependent variable, and factors like reactant concentration and pressure are maintained as control variables. This meticulous control allows for clear interpretation of the impact of temperature on the reaction kinetics. Careful attention to these variables strengthens the validity and reliability of the experimental findings.
Experimentation bridges the gap between observation and understanding, providing a framework for testing hypotheses and drawing evidence-based conclusions about the nature of chemical and physical changes. Rigorous experimental design ensures that collected data accurately reflect the underlying processes being investigated. Challenges in experimentation often arise from the difficulty in controlling all variables perfectly, particularly in complex systems. Addressing these challenges requires careful experimental design, replication of experiments, and statistical analysis to account for variability and uncertainty. Overcoming these challenges strengthens the conclusions drawn from the experimental results and contributes to a deeper understanding of the intricate processes governing matter transformations.
4. Analysis
Analysis represents a crucial stage in any chemical and physical changes project, bridging the gap between raw data and meaningful conclusions. It involves scrutinizing collected data, identifying patterns, and drawing inferences about the underlying processes governing the observed transformations. Analysis provides the framework for interpreting experimental results, connecting empirical observations to theoretical understanding. For instance, analyzing the mass of reactants and products in a chemical reaction allows for the determination of stoichiometric relationships and the assessment of percent yield, providing insights into the efficiency of the reaction. Similarly, analyzing the temperature change during a physical change, such as melting ice, offers insights into the energy transfer associated with the phase transition. The depth of analysis directly influences the strength and validity of the project’s conclusions.
Effective analysis often involves multiple approaches, including calculations, graphing, and statistical analysis. Calculations enable the quantification of observed changes, such as determining the rate of a reaction or the amount of heat absorbed or released. Graphing facilitates visualization of trends and relationships within the data, aiding in the identification of patterns and anomalies. Statistical analysis provides tools for assessing the reliability of the data and determining the significance of observed differences. For example, calculating the slope of a graph plotting reactant concentration versus time provides a quantitative measure of reaction rate, while statistical tests can determine whether observed differences in reaction rates under different conditions are statistically significant. The appropriate analytical techniques depend on the specific research questions and the nature of the collected data. Integrating these diverse analytical tools provides a comprehensive understanding of the observed transformations.
Thorough analysis is essential for extracting meaningful insights from the data collected during a chemical and physical changes project. It provides the foundation for drawing evidence-based conclusions, connecting experimental observations to underlying scientific principles. Challenges in analysis often arise from the complexity of the data or the presence of confounding factors. Addressing these challenges requires careful consideration of experimental design, appropriate selection of analytical methods, and rigorous interpretation of results. Overcoming these challenges enhances the accuracy and reliability of the project’s findings, contributing to a deeper understanding of the intricate processes governing matter transformations. The quality of the analysis directly impacts the overall scientific merit of the project.
5. Documentation
Documentation serves as a cornerstone of any robust chemical and physical changes project, providing a comprehensive record of the entire scientific process. Thorough documentation encompasses all aspects of the investigation, from initial experimental design and data collection to analysis and interpretation of results. This meticulous record-keeping ensures transparency, reproducibility, and facilitates effective communication of findings. Documentation establishes a verifiable account of the project’s progression, enabling scrutiny, validation, and future replication of the work. For instance, documenting the precise concentrations of reactants used in a chemical reaction allows other researchers to reproduce the experiment and verify the results. Similarly, documenting the calibration procedures for instruments used in data collection enhances the credibility and reliability of the measurements. Without comprehensive documentation, the scientific value of the project diminishes significantly. The quality of documentation directly impacts the project’s credibility and potential contribution to the scientific community. Effective documentation allows for retrospective analysis, enabling researchers to identify potential sources of error, refine experimental procedures, and build upon previous work.
Maintaining a detailed laboratory notebook constitutes a central component of proper documentation. This notebook should contain a chronological record of all experimental procedures, observations, data, and calculations. Each entry should be dated and signed, providing a clear audit trail of the project’s development. The notebook serves as a primary source of information, enabling researchers to trace the evolution of the project, understand the rationale behind decisions made, and interpret the results in context. Including detailed descriptions of experimental setups, instrument settings, and any deviations from planned procedures ensures that the experiment can be accurately replicated. For example, documenting the specific type of filter paper used in a filtration experiment might seem trivial, but it can significantly influence the outcome if the filter paper’s properties affect the filtration rate or the purity of the filtrate. Careful attention to these seemingly minor details enhances the reproducibility and scientific rigor of the project. The laboratory notebook becomes a valuable resource for both the researchers conducting the project and the broader scientific community.
In conclusion, thorough documentation stands as an indispensable element of any credible chemical and physical changes project. It provides a transparent and verifiable record of the scientific process, facilitating reproducibility, communication, and future research. Challenges in documentation can arise from time constraints or the perceived burden of meticulous record-keeping. However, recognizing the crucial role of documentation in ensuring the integrity and value of scientific research underscores the importance of prioritizing this aspect of the project. Effective documentation strengthens the project’s contribution to the scientific community, enabling scrutiny, validation, and the advancement of scientific knowledge. The comprehensiveness of the documentation reflects the rigor and reliability of the scientific investigation.
Frequently Asked Questions
Addressing common inquiries regarding investigations into matter transformations can clarify conceptual understanding and enhance experimental design. The following questions and answers provide further insights into these scientific explorations.
Question 1: What distinguishes a chemical change from a physical change?
A chemical change involves the formation of new substances with different chemical properties, while a physical change alters the form or state of a substance without changing its chemical composition. Chemical changes involve the breaking and forming of chemical bonds, whereas physical changes typically involve alterations in intermolecular forces.
Question 2: How can one ascertain whether a gas produced during an experiment signifies a chemical change?
Gas evolution alone does not definitively indicate a chemical change. Further analysis is required to determine if the gas produced has a different chemical composition from the original substances. If the evolved gas represents a new substance formed through chemical reaction, then it indicates a chemical change. If the gas is simply a dissolved gas being released due to a change in temperature or pressure, it represents a physical change.
Question 3: Are all color changes indicative of chemical transformations?
Not all color changes signify chemical transformations. Mixing different colored paints, for instance, produces a new color but doesn’t alter the chemical composition of the paints; its a physical change. A color change resulting from a chemical reaction, like the browning of an apple upon exposure to air (oxidation), signifies a chemical transformation.
Question 4: How does understanding transformations of matter benefit practical applications?
Understanding these transformations is crucial for advancements in various fields. Developing new materials, optimizing industrial processes, understanding environmental phenomena, and diagnosing medical conditions all rely on a fundamental understanding of how matter interacts and transforms. This knowledge underpins technological innovation and scientific discovery across disciplines.
Question 5: What are some common misconceptions about physical and chemical changes?
A common misconception is that physical changes are always reversible, while chemical changes are irreversible. While many physical changes are easily reversible (e.g., melting and freezing of water), some are not (e.g., shattering glass). Similarly, some chemical changes can be reversed under specific conditions. Another misconception is that all chemical changes are rapid and dramatic. Corrosion, for instance, is a slow chemical change. Clarity requires focusing on the formation of new substances, not just the speed or visibility of the transformation.
Question 6: How does experimental design impact the reliability of conclusions in investigations of matter transformations?
Experimental design directly influences the validity and reliability of conclusions. Careful control of variables isolates the effect of the investigated factor. Proper replication and statistical analysis ensure that observed changes are not due to random chance. A robust experimental design strengthens the cause-and-effect relationship established between the manipulated variables and the observed outcomes, leading to more reliable and scientifically sound conclusions.
Understanding the fundamental principles of matter transformations is essential for scientific literacy. These principles underpin numerous natural phenomena and technological advancements. Further exploration of specific transformations and experimental techniques can deepen this understanding.
The following section will explore specific examples of chemical and physical changes commonly encountered in experimental settings.
Conclusion
Systematic investigations of matter transformations, encompassing meticulous observation, precise classification, rigorous experimentation, thorough analysis, and comprehensive documentation, provide crucial insights into the fundamental principles governing the natural world. Understanding the distinction between physical changes, which alter form without changing composition, and chemical changes, which result in new substances, is essential for interpreting observed phenomena. Effective experimental design, incorporating careful control of variables, enables researchers to establish cause-and-effect relationships and draw valid conclusions. Rigorous analysis of collected data, employing appropriate analytical techniques, reveals patterns and trends, leading to a deeper understanding of the underlying processes. Meticulous documentation ensures transparency, reproducibility, and facilitates communication of findings, contributing to the advancement of scientific knowledge.
Continued exploration of chemical and physical changes remains essential for progress in diverse scientific disciplines. From developing novel materials to understanding complex biological processes, these fundamental principles underpin countless technological innovations and scientific discoveries. A deeper understanding of these transformations empowers informed decision-making regarding resource management, environmental protection, and technological advancement. Further research and investigation are crucial for addressing ongoing challenges and unlocking future possibilities in the realm of scientific understanding and its practical applications.