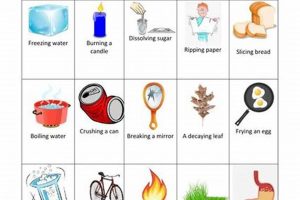
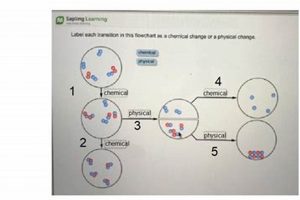
Chemical changes involve alterations in the composition of matter, resulting in new substances with different properties. Physical changes, conversely, alter the form or state of matter without affecting its chemical composition. Consider burning wood. This combustion creates ash and releases gases, a chemical transformation. Simply chopping wood alters its shape but leaves its chemical makeup unchanged, illustrating a physical change. Reversing the chemical change of combustion through a physical process like cooling or applying pressure to the resulting ash and gases will not reconstitute the original wood. The fundamental chemical bonds have been broken and reformed into different substances.
Understanding the distinction between these two types of changes is crucial in numerous scientific fields, including chemistry, materials science, and engineering. This distinction enables scientists to manipulate materials effectively, designing processes for synthesis, separation, and analysis. Historically, the ability to differentiate between these changes marked a significant advancement in chemical understanding, paving the way for modern chemical theory and practice. Without this foundational knowledge, advancements like pharmaceutical development or the creation of novel materials would be severely hindered.
This fundamental principle underlies various critical concepts in science and technology. The following sections will explore specific examples further illustrating the irreversible nature of chemical changes and delve into the limitations of physical processes in reversing them. Additionally, the discussion will encompass related topics such as the law of conservation of mass and the energy changes associated with chemical transformations.
Understanding the Irreversibility of Chemical Reactions
Manipulating matter requires a clear understanding of the distinction between chemical and physical changes. The following tips provide guidance on differentiating these changes and highlight the limitations of reversing chemical transformations through physical processes.
Tip 1: Consider Compositional Changes: Chemical changes inherently alter the composition of matter, resulting in new substances with different properties. Physical changes do not alter composition, only the form or state of the substance.
Tip 2: Observe Energy Transfer: Chemical reactions typically involve significant energy changes, either releasing (exothermic) or absorbing (endothermic) energy. Physical changes often involve smaller energy transfers related to phase transitions.
Tip 3: Analyze the Reversibility: Physical changes are generally easily reversible, often by simply reversing the initial action (e.g., melting and freezing). Chemical changes are not easily reversed, and reversing them requires other chemical reactions.
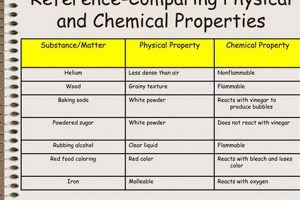
Tip 4: Examine the Products: In a chemical change, the products exhibit different chemical properties than the reactants. In physical changes, the substance retains its original properties.
Tip 5: Recognize Examples of Chemical Changes: Combustion, oxidation, and decomposition are clear indicators of chemical transformations. Dissolving, melting, and boiling are typical physical changes.
Tip 6: Note the Role of Physical Processes in Separating Mixtures: While physical processes cannot reverse chemical changes, they can be used to separate the products of a chemical reaction, leveraging differences in physical properties.
Grasping these key differences is essential for informed decision-making in scientific endeavors. Understanding these concepts facilitates the efficient design and manipulation of materials and processes.
By internalizing these principles, a deeper appreciation of the intricacies of matter manipulation can be achieved, paving the way for further exploration of chemical principles and applications.
1. Chemical Bonds Altered
Chemical bonds, the forces holding atoms together within molecules, play a central role in distinguishing chemical changes from physical ones. When a chemical change occurs, existing chemical bonds are broken, and new bonds are formed, resulting in different substances with distinct properties. This rearrangement of atoms and the associated energy changes are fundamental to the nature of chemical reactions. Physical changes, however, do not involve breaking or forming chemical bonds. Instead, they affect the intermolecular forces between molecules, altering the state or form of a substance without changing its chemical composition.
The alteration of chemical bonds is a defining characteristic of chemical reactions and directly impacts the irreversibility of these changes through physical means. Consider the combustion of methane (CH). During this reaction, the C-H bonds in methane and the O=O bonds in oxygen are broken, while new C=O bonds in carbon dioxide (CO) and O-H bonds in water (HO) are formed. This fundamental change in bonding makes it impossible to reverse the reaction simply by applying physical processes like cooling or compression. To recover methane and oxygen, new chemical reactions would be required to break the C=O and O-H bonds and reform the original C-H and O=O bonds. This principle extends to other chemical reactions, highlighting the crucial role of bond alteration in determining the irreversibility of chemical changes.
The understanding that chemical bond alteration signifies a chemical change has profound practical implications. It informs the design and optimization of chemical processes, from industrial synthesis to pharmaceutical development. Recognizing the permanence of these bond changes underscores the need for careful consideration of reactants, reaction conditions, and potential byproducts. Furthermore, this knowledge is essential for developing strategies to mitigate the environmental impact of chemical processes, emphasizing the importance of designing sustainable and reversible chemical transformations wherever possible.
2. New Substances Formed
The formation of new substances is a defining characteristic of chemical changes and a key factor in their irreversibility through physical processes. When new substances are formed, the original reactants are fundamentally transformed, resulting in products with distinct chemical and physical properties. This transformation involves the breaking and forming of chemical bonds, leading to a rearrangement of atoms and a change in the overall composition of matter. This contrasts sharply with physical changes, which only alter the form or state of a substance without affecting its underlying chemical identity.
- Distinct Chemical Properties
New substances formed in chemical reactions exhibit distinct chemical properties compared to the original reactants. These properties, which dictate how a substance interacts with other substances, arise from the unique arrangement of atoms and bonds within the molecule. For instance, burning magnesium metal in air produces magnesium oxide, a white powder with different chemical properties than the original shiny, metallic magnesium. The change in properties reflects the formation of new chemical bonds and the creation of a new substance. This alteration of chemical identity underscores the irreversible nature of chemical changes through physical processes.
- Different Physical Properties
In addition to distinct chemical properties, new substances formed in chemical reactions also exhibit different physical properties. These properties, such as melting point, boiling point, density, and color, are influenced by the arrangement of molecules and the intermolecular forces between them. The formation of magnesium oxide, for example, results in a substance with a much higher melting point and a different color than the original magnesium metal. These changes in physical properties are a direct consequence of the formation of a new substance with a different molecular structure. Physical processes, such as heating or cooling, can alter the physical state of a substance, but they cannot reverse the chemical transformation and restore the original reactants.
- Irreversibility Through Physical Means
The formation of new substances with distinct chemical and physical properties highlights the fundamental reason why chemical changes cannot be reversed by physical processes. Physical processes operate on the level of intermolecular forces, affecting the state or form of a substance without altering its chemical composition. However, reversing a chemical change requires breaking and reforming chemical bonds to recreate the original reactants. This cannot be achieved through physical manipulation alone. For instance, separating the magnesium and oxygen atoms in magnesium oxide requires a chemical reaction, not simply a physical separation technique.
- Implications for Chemical Processes
The understanding that new substances formed in chemical reactions possess distinct properties has significant implications for various chemical processes. It underscores the importance of carefully controlling reaction conditions to obtain desired products and minimize unwanted byproducts. Furthermore, this knowledge informs the development of separation and purification techniques, which rely on differences in physical properties to isolate and purify specific substances from complex mixtures. It also emphasizes the importance of proper waste management and disposal practices, recognizing that chemical transformations often result in substances with potentially hazardous properties.
In essence, the formation of new substances with unique chemical and physical properties underscores the irreversible nature of chemical changes with respect to physical processes. This fundamental principle has far-reaching implications in numerous fields, from materials science and chemical engineering to environmental science and medicine, highlighting the importance of understanding the distinction between chemical and physical transformations.
3. Physical processes insufficient
The insufficiency of physical processes to reverse chemical changes stems from the fundamental difference between these two types of transformations. Chemical changes involve alterations in the composition of matter, specifically the breaking and forming of chemical bonds, leading to the creation of new substances with distinct properties. Physical processes, on the other hand, affect only the physical state or form of a substance without altering its chemical composition. Since they do not affect chemical bonds, physical processes like heating, cooling, or applying pressure cannot reverse the bond rearrangements that define a chemical change. For instance, consider the rusting of iron. This chemical change involves the reaction of iron with oxygen to form iron oxide. Simply grinding the rust (iron oxide) into a finer powder, a physical process, will not revert it back to iron and oxygen. The iron-oxygen bonds remain intact. This underscores the principle that reversing a chemical change requires another chemical reaction to break and reform bonds, something physical processes cannot achieve.
The practical implications of this principle are substantial. In industrial settings, understanding the limitations of physical processes in reversing chemical reactions is crucial for process design and optimization. For example, in the production of pharmaceuticals, separating the desired product from byproducts often necessitates chemical methods rather than simply physical separations. Similarly, in environmental remediation, physical processes like filtration or adsorption might remove pollutants, but they may not necessarily reverse the chemical changes that created those pollutants. Chemical treatments are often required for true remediation. Furthermore, this understanding is fundamental to the design of sustainable chemical processes, emphasizing the importance of designing reactions that are either inherently reversible or that produce byproducts that can be easily converted back to valuable reactants through further chemical reactions.
In summary, the inability of physical processes to reverse chemical changes lies in the distinct nature of these transformations. Chemical changes involve alterations in chemical bonds and the formation of new substances, while physical processes only affect the physical state or form of matter without changing its composition. This fundamental difference dictates that reversing a chemical change requires another chemical reaction, highlighting the limitations of physical processes in this context. This understanding has broad practical implications in diverse fields, from industrial chemistry and environmental science to materials science and medicine.
4. Reversibility requires chemical means
The statement “Reversibility requires chemical means” directly addresses the core question of whether a chemical change can be reversed by a physical change. It establishes a fundamental principle: reversing a chemical change necessitates altering the chemical bonds and composition of the newly formed substances. This inherently requires chemical processes, not simply physical manipulations like heating, cooling, or changes in pressure. Physical changes, by definition, do not alter the chemical composition of matter. They merely affect the physical state or form. Therefore, they are inherently insufficient to reverse the bond rearrangements that define a chemical change. For instance, the combustion of hydrogen with oxygen produces water. While boiling or freezing water (physical changes) can alter its state, they cannot revert it back to hydrogen and oxygen. This requires another chemical reaction, such as electrolysis, which uses electrical energy to break the chemical bonds in water molecules.
The practical significance of this understanding is far-reaching. In materials science, it informs the design of new materials and processes, recognizing that altering material properties at the molecular level requires chemical interventions. In environmental science, it highlights the limitations of physical remediation methods for chemical pollutants. Cleaning up oil spills, for instance, may involve physical containment and removal, but ultimately, breaking down the oil components requires chemical processes. In chemical engineering, this principle guides the development of efficient and sustainable chemical processes, emphasizing the need for reactions that are either inherently reversible or that produce byproducts easily convertible back to valuable reactants through further chemical reactions. The Haber-Bosch process, used to synthesize ammonia, exemplifies this, where careful control of temperature and pressure optimizes the reaction yield while allowing for some reversibility.
In summary, the principle that “reversibility requires chemical means” underscores the fundamental distinction between chemical and physical changes. It provides a crucial framework for understanding why physical processes cannot reverse chemical transformations. This understanding is essential across diverse scientific and engineering disciplines, informing the design and optimization of processes ranging from materials synthesis to environmental remediation. The challenges in reversing certain chemical changes also highlight the importance of designing sustainable chemical processes that minimize waste and maximize resource utilization, emphasizing the need for further research into catalytic and reversible reactions.
5. Energy Changes Significant
Significant energy changes are a hallmark of chemical reactions and play a crucial role in understanding why physical processes cannot reverse them. Chemical changes involve the breaking and forming of chemical bonds, which are inherently associated with energy transfers. These energy changes represent a fundamental difference between chemical and physical transformations. Physical changes typically involve smaller energy transfers associated with changes in state, such as melting or boiling, while chemical changes often involve substantial releases or absorptions of energy.
- Exothermic Reactions and Irreversibility
Exothermic reactions release energy, often in the form of heat or light. Combustion reactions, such as burning wood or methane, are prime examples. The energy released during these reactions is a direct consequence of the formation of stronger chemical bonds in the products compared to the reactants. This significant energy release makes it thermodynamically unfavorable to reverse the reaction through physical means. The energy released would need to be re-supplied, and simply cooling the products will not reconstitute the original reactants.
- Endothermic Reactions and Energy Requirements
Endothermic reactions absorb energy from the surroundings. Photosynthesis, the process by which plants convert sunlight into chemical energy, is a classic example. These reactions require a continuous input of energy to proceed. While some endothermic reactions can be reversed, doing so requires supplying the energy absorbed during the forward reaction. Physical processes like increasing pressure or changing temperature alone are insufficient to reverse the chemical transformation. A specific energy input, equivalent to the energy absorbed in the forward reaction, is necessary.
- Energy Barriers and Activation Energy
Even in seemingly reversible chemical reactions, an energy barrier, known as the activation energy, must be overcome for the reaction to proceed in either direction. This activation energy represents the minimum energy required to break the existing bonds and initiate the reaction. Physical processes like heating can sometimes provide enough energy to overcome the activation energy barrier in one direction, but they typically cannot simultaneously reverse the reaction by overcoming the activation energy barrier in the opposite direction. Specific catalysts or other chemical interventions may be required to lower the activation energy and facilitate reversibility.
- The Role of Thermodynamics
The laws of thermodynamics govern the direction and extent of chemical reactions. The second law, in particular, states that spontaneous processes tend to increase the entropy (disorder) of the universe. Many chemical reactions are spontaneous and proceed with a decrease in free energy, making them thermodynamically favorable. Reversing such reactions requires overcoming this thermodynamic driving force, which necessitates a specific energy input or a coupled reaction that provides the necessary free energy change. Physical processes alone cannot achieve this.
In conclusion, the significant energy changes associated with chemical reactions are a key factor in their irreversibility through physical means. Whether a reaction releases energy (exothermic) or absorbs energy (endothermic), the magnitude of these energy changes underscores the fundamental difference between chemical and physical transformations. Reversing a chemical change requires not only manipulating physical conditions but also addressing the underlying energetic and thermodynamic factors governing the reaction. This often necessitates chemical interventions, such as providing specific energy inputs, using catalysts, or coupling the reverse reaction with another energetically favorable process. The interplay between energy changes and chemical transformations is central to understanding the limitations of physical processes in reversing chemical reactions and highlights the importance of considering both energy and entropy in the design and manipulation of chemical systems.
Frequently Asked Questions
This section addresses common queries regarding the reversibility of chemical changes, clarifying the distinction between chemical and physical transformations.
Question 1: Can dissolving salt in water be reversed by evaporation?
While evaporation removes the water, leaving the salt behind, this is a physical separation, not a reversal of a chemical change. Dissolving salt is generally considered a physical change as the salt retains its chemical identity and can be recovered by physical means.
Question 2: Is melting ice a chemical change? Why or why not?
Melting ice is a physical change. The chemical composition of water remains the same, HO, in both solid (ice) and liquid states. The change affects only the arrangement and interaction of water molecules, not their chemical identity.
Question 3: If burning paper produces ash, can the ash be turned back into paper?
No. Burning paper is a chemical change where the cellulose in paper reacts with oxygen to produce ash, smoke, and gases. These products have different chemical compositions than the original paper. Physical processes cannot reconstruct the complex cellulose molecules.
Question 4: How does the concept of chemical bonding relate to the irreversibility of chemical changes?
Chemical changes involve breaking and forming chemical bonds, resulting in new substances with different compositions. Physical processes do not alter these bonds. Therefore, they cannot reverse the fundamental changes in composition that occur during a chemical reaction.
Question 5: Are all chemical changes irreversible? Are there exceptions?
While many chemical changes are practically irreversible under normal conditions, some are reversible under specific conditions. Reversible reactions reach a state of equilibrium where the forward and reverse reactions occur at equal rates. However, even in reversible reactions, the fundamental principle remains: reversing a chemical change requires another chemical reaction.
Question 6: Why is understanding the difference between chemical and physical changes important in practical applications?
Distinguishing between these changes is crucial in various fields. In materials science, it guides material design and processing. In environmental science, it informs remediation strategies. In chemical engineering, it influences process optimization and the development of sustainable chemical technologies.
The consistent theme throughout these FAQs emphasizes the fundamental difference between chemical and physical changes and the limitations of physical processes in reversing chemical transformations. Understanding this principle is crucial for scientific literacy and for developing effective strategies in diverse fields.
The next section will delve further into the energetic and thermodynamic factors that govern chemical changes and influence their reversibility.
Can a Chemical Change Be Reversed by a Physical Change? A Conclusive Perspective
The exploration of whether a chemical change can be reversed by a physical change reveals a fundamental principle in the natural sciences: chemical and physical transformations operate at distinct levels. Chemical changes involve alterations in the composition of matter, specifically the breaking and forming of chemical bonds, leading to new substances with distinct properties. Physical changes, conversely, affect only the physical state or form of a substance without altering its chemical composition. This core difference dictates that reversing a chemical change necessitates another chemical reaction to rearrange the atoms and bonds, a feat impossible through mere physical manipulation like heating, cooling, or pressure changes. The significant energy changes accompanying chemical reactions further underscore this distinction, as the energy released or absorbed during bond breaking and formation represents a thermodynamic barrier that physical processes alone cannot overcome.
The implications of this principle are profound and far-reaching. From designing sustainable chemical processes and developing effective remediation strategies for environmental pollutants to advancing materials science and understanding biological systems, the distinction between chemical and physical changes provides an essential framework for scientific inquiry and technological innovation. Further research into catalytic processes, reversible reactions, and the manipulation of energy at the molecular level holds the key to developing more efficient, sustainable, and potentially reversible chemical transformations in the future. A continued appreciation and deeper exploration of this fundamental principle will be crucial for advancing scientific understanding and addressing critical challenges in diverse fields.