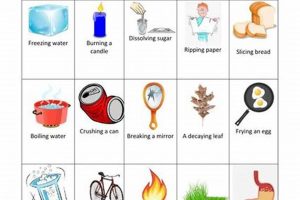
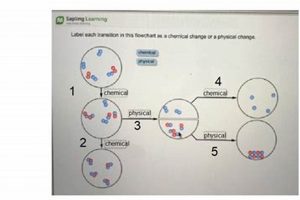
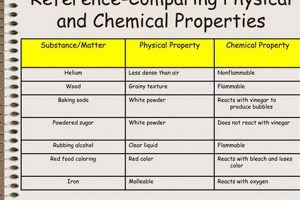
A laboratory setting designed for the observation and analysis of matter’s characteristics often involves investigating traits like density, melting point, and conductivity, alongside reactivity with other substances, such as combustion or oxidation. Practical experiments might include measuring the volume and mass of a solid to calculate its density, observing a substance’s behavior when heated or cooled, or testing its reaction with various reagents.
Such investigations are fundamental to scientific understanding. They provide the foundational knowledge for fields like material science, chemistry, and engineering, enabling the development of new materials and technologies. Historically, these types of analyses were crucial for alchemists and early chemists, forming the basis of modern chemical theory. A strong grasp of these concepts allows for informed predictions about how different substances will interact and behave under specific conditions.
The following sections will explore specific experimental methods, common materials used in such analyses, and the safety procedures necessary for conducting these experiments effectively and safely.
Tips for Effective Laboratory Practices
Careful preparation and execution are crucial for obtaining reliable data and ensuring safety when investigating material characteristics.
Tip 1: Accurate Measurements: Precise measurements of mass, volume, and temperature are essential for accurate calculations of properties like density and specific heat. Employ appropriate instruments and techniques for each measurement, considering significant figures and potential sources of error.
Tip 2: Controlled Environments: External factors like temperature and pressure can influence experimental outcomes. Maintaining consistent environmental conditions throughout the procedure is essential for reliable results.
Tip 3: Proper Handling of Chemicals: Many substances pose hazards. Consult safety data sheets (SDS) before handling any chemical and adhere to established safety protocols, including wearing appropriate personal protective equipment (PPE).
Tip 4: Observation and Documentation: Careful observation and meticulous recording of all experimental data, including both qualitative and quantitative observations, are fundamental for accurate analysis and interpretation.
Tip 5: Data Analysis and Interpretation: Analyze collected data using appropriate methods, including calculations, graphs, and statistical analysis. Draw conclusions based on evidence and consider potential sources of error.
Tip 6: Cleanliness and Organization: A clean and organized workspace promotes efficiency and reduces the risk of accidents or contamination. Ensure all equipment is clean and properly stored before and after use.
Tip 7: Waste Disposal: Dispose of chemical waste according to established procedures and regulations to minimize environmental impact and maintain a safe laboratory environment.
Adhering to these practices ensures data integrity, promotes safety, and contributes to a deeper understanding of material behavior.
By following these guidelines, investigations can be conducted efficiently and safely, yielding reliable results that contribute to a comprehensive understanding of the properties of matter.
1. Observation
Observation forms the bedrock of scientific inquiry, particularly within a laboratory setting dedicated to analyzing physical and chemical properties. It serves as the initial step in the scientific method, driving hypothesis formation and experimental design. Careful observation provides qualitative data that informs subsequent quantitative measurements and analyses.
- Qualitative Observations
Qualitative observations describe characteristics without numerical values. Examples include noting the color of a precipitate, the odor of a gas, or the texture of a material. In the context of material analysis, qualitative observations provide crucial preliminary information, often guiding subsequent quantitative measurements. For example, observing the effervescence of a substance upon adding acid suggests a chemical reaction producing gas, prompting further investigation to identify the gas and quantify its production.
- Quantitative Observations
Quantitative observations involve numerical measurements. These observations employ instruments to provide precise data about properties like mass, volume, temperature, and absorbance. In a physical and chemical properties lab, quantitative observations are essential for calculating density, determining melting point, and quantifying reaction rates. For example, measuring the volume of gas produced over time allows for the determination of reaction kinetics.
- Changes Over Time
Observing how properties change over time offers insights into dynamic processes. Monitoring temperature fluctuations during a reaction, tracking the rate of precipitate formation, or observing color changes provides valuable data for understanding reaction mechanisms and kinetics. For example, observing the gradual color change of a solution during titration allows for precise determination of the equivalence point.
- Instrumentation and Tools
The tools used for observation directly influence the quality and type of data obtained. Microscopes reveal microscopic details, spectrometers analyze light interactions, and balances measure mass with precision. Selecting appropriate instrumentation is critical for obtaining relevant and accurate data. For example, using a high-resolution microscope allows observation of crystal structure, informing analysis of material properties.
These interconnected facets of observation highlight its critical role in the characterization of materials. Careful observation, whether qualitative or quantitative, provides the foundation for deeper analysis, leading to a more comprehensive understanding of matter’s physical and chemical properties within a laboratory setting. From initial visual assessments to precise instrumental measurements, observation informs every stage of scientific investigation.
2. Measurement
Measurement forms the quantitative backbone of any physical and chemical properties lab. It provides the numerical data essential for characterizing materials and understanding their behavior. Accurate and precise measurements are crucial for drawing meaningful conclusions about material properties, enabling comparisons, and formulating scientific laws. The relationship between measurement and material analysis is one of dependence; accurate analysis relies entirely on the quality of the underlying measurements.
Consider the determination of density. This fundamental property requires precise measurements of mass and volume. An error in either measurement directly impacts the calculated density, potentially leading to misidentification of a substance or incorrect predictions about its behavior. Similarly, determining the melting point of a solid necessitates careful temperature monitoring. Precise temperature measurements allow for accurate identification of the phase transition and provide insights into the material’s intermolecular forces. In chemical reactions, measuring the volume of gas produced over time quantifies reaction rates, offering valuable kinetic data. These examples illustrate how measurements of various physical and chemical properties are intertwined with analytical outcomes.
The practical implications of precise measurement extend beyond the laboratory. In industrial settings, accurate measurements are crucial for quality control, ensuring product consistency and performance. For example, in pharmaceutical manufacturing, precise measurements of reactants are essential for drug efficacy and safety. In material science, precise measurements of tensile strength and elasticity guide the development of new materials with specific properties. Therefore, understanding the fundamental role of measurement in a physical and chemical properties lab provides a foundation for numerous scientific and technological applications. Challenges in accurate measurement can arise from limitations in instrumentation, environmental fluctuations, and human error. Addressing these challenges through careful calibration, controlled environments, and rigorous experimental procedures enhances the reliability and validity of experimental results, ultimately contributing to a more robust understanding of matter.
3. Experimentation
Experimentation lies at the heart of a physical and chemical properties lab. It provides the framework for systematically investigating matter, testing hypotheses, and gathering empirical data. Well-designed experiments isolate variables, allowing for the determination of cause-and-effect relationships and the elucidation of fundamental principles governing material behavior. The experimental process serves as a bridge between observation and understanding, transforming raw data into meaningful insights about the properties of matter.
- Hypothesis Testing
Experiments are designed to test specific hypotheses about material properties. For example, one might hypothesize that increasing temperature increases reaction rate. A controlled experiment would then systematically vary temperature while keeping other factors constant, measuring the reaction rate at each temperature point. The results would either support or refute the initial hypothesis, advancing understanding of the relationship between temperature and reaction kinetics. This rigorous approach is crucial for establishing valid scientific conclusions.
- Variable Control
Controlling variables is paramount in experimentation. In investigating the effect of concentration on reaction rate, all factors other than concentration (e.g., temperature, pressure, catalyst presence) must remain constant. This isolates the effect of concentration, allowing for clear attribution of observed changes in reaction rate to changes in concentration. Careful control of variables ensures that experimental results reflect the intended manipulation, minimizing confounding factors.
- Data Collection and Analysis
Experimentation involves systematic data collection using appropriate instruments and techniques. Data can be qualitative (e.g., color change, precipitate formation) or quantitative (e.g., temperature readings, mass measurements). Collected data is then analyzed using statistical methods to identify trends, patterns, and relationships between variables. This analysis forms the basis for drawing conclusions and formulating scientific explanations. For example, measuring the pH of a solution at different time points provides data for analyzing reaction progress and determining equilibrium constants.
- Reproducibility
Reproducibility is a cornerstone of scientific validity. Experimental procedures must be documented meticulously, allowing other researchers to replicate the experiment and verify the results. Reproducibility builds confidence in the reliability of findings and strengthens scientific understanding. The ability to reproduce experimental results under different conditions validates the robustness of the observed phenomena and contributes to the establishment of scientific laws.
These facets of experimentation underscore its importance in a physical and chemical properties lab. From formulating testable hypotheses to analyzing collected data and ensuring reproducibility, experimentation provides the structured framework for advancing our understanding of matter. Through controlled investigation, experimentation allows us to unravel the complexities of material behavior, leading to the discovery of new properties and the development of innovative applications.
4. Analysis
Analysis forms the interpretive bridge between raw data and scientific understanding within a physical and chemical properties lab. It transforms observations and measurements into meaningful insights, revealing underlying patterns, relationships, and principles governing material behavior. Without rigorous analysis, experimental data remains a collection of numbers and observations, devoid of explanatory power. Analysis provides the framework for extracting knowledge and drawing conclusions from experimental findings.
- Data Interpretation
Data interpretation involves scrutinizing experimental results to identify trends, patterns, and anomalies. This may involve constructing graphs, performing statistical analyses, and comparing data to theoretical predictions. For example, analyzing a graph of reaction rate versus temperature reveals the relationship between these variables and provides insights into the activation energy of the reaction. Interpreting spectroscopic data allows for the identification of functional groups within a molecule, elucidating its chemical structure. Accurate data interpretation is crucial for drawing valid conclusions about material properties.
- Error Analysis
All measurements are subject to error. Error analysis quantifies the uncertainty associated with experimental data, providing a measure of its reliability. Understanding potential sources of error, both systematic and random, allows for a more nuanced interpretation of results and informs future experimental design. For example, calculating the standard deviation of multiple measurements provides a measure of precision. Identifying systematic errors, such as instrumental bias, allows for corrections and improvements in experimental methodology.
- Correlation and Causation
Analysis distinguishes between correlation and causation. Observing a correlation between two variables does not necessarily imply a causal relationship. Further investigation and controlled experiments are often required to establish causality. For example, observing a correlation between the concentration of a substance and reaction rate does not definitively prove that concentration changes cause the observed rate changes. Controlled experiments manipulating concentration while holding other factors constant are needed to establish a causal link.
- Model Development
Analysis often involves developing models to explain observed phenomena. Models can be mathematical equations, conceptual frameworks, or simulations. Models provide a simplified representation of reality, allowing for predictions and further testing of hypotheses. For example, developing a kinetic model for a chemical reaction allows for prediction of reaction rates under different conditions. Comparing model predictions with experimental data refines the model and enhances understanding of the underlying chemical processes.
These facets of analysis highlight its crucial role in extracting meaning from experimental data within a physical and chemical properties lab. From interpreting trends to evaluating error and developing explanatory models, analysis transforms raw data into scientific knowledge. This analytical process, applied rigorously, enhances our understanding of material behavior, contributing to advancements in various scientific disciplines and technological applications.
5. Interpretation
Interpretation represents the culmination of the scientific process within a physical and chemical properties lab. It is the stage where experimental findings are synthesized, contextualized, and translated into meaningful conclusions about the nature of matter. Interpretation bridges the gap between empirical data and scientific understanding, providing the framework for explaining observed phenomena and predicting future behavior. Without careful interpretation, experimental results remain isolated pieces of information, lacking broader scientific significance.
- Connecting Data to Theory
Interpretation links experimental data to established scientific theories and principles. Observed trends and patterns are explained in terms of underlying chemical and physical laws. For example, the observed relationship between temperature and reaction rate can be interpreted using collision theory, which postulates that increased temperature leads to more frequent and energetic collisions between reactant molecules. Connecting experimental findings to existing theoretical frameworks strengthens scientific understanding and allows for broader generalizations.
- Drawing Conclusions and Making Predictions
Interpretation involves drawing conclusions based on the totality of evidence gathered. These conclusions address the initial research questions and hypotheses that motivated the experiment. Furthermore, interpretation allows for predictions about future behavior under different conditions. For example, based on the observed relationship between pressure and gas volume, one can predict the volume of a gas at a different pressure. Accurate predictions validate experimental findings and demonstrate the predictive power of scientific understanding.
- Identifying Limitations and Sources of Uncertainty
Interpretation acknowledges the inherent limitations of experimental data and the potential for uncertainty. Experimental error, limitations in instrumentation, and the complexity of real-world systems can all contribute to uncertainty in findings. Acknowledging these limitations strengthens the integrity of scientific conclusions and informs future research directions. For instance, recognizing the limitations of a particular analytical technique in measuring trace amounts of a substance clarifies the scope of the conclusions that can be drawn.
- Communicating Findings
Interpretation culminates in the clear and concise communication of findings to the scientific community. This communication typically involves publishing research papers, presenting at conferences, and engaging in scientific discourse. Effective communication disseminates knowledge, fosters collaboration, and contributes to the advancement of scientific understanding. Clear articulation of experimental results, their interpretation, and their implications is essential for furthering scientific progress.
These interconnected facets of interpretation highlight its critical role in transforming data into knowledge within a physical and chemical properties lab. By connecting data to theory, drawing conclusions, acknowledging limitations, and communicating findings effectively, interpretation contributes to a deeper understanding of the properties of matter and advances scientific progress. This final stage of the scientific process ensures that experimental findings are not merely isolated observations, but rather integrated components of a larger scientific narrative, contributing to the ongoing exploration of the physical and chemical world.
6. Safety
Safety is paramount in any environment involving the investigation of physical and chemical properties. Laboratories dedicated to this purpose often contain hazardous materials and equipment, necessitating stringent safety protocols to prevent accidents and ensure the well-being of all personnel. A comprehensive understanding of potential hazards and appropriate safety measures is not merely a prerequisite but an integral component of successful laboratory practice. Negligence in this area can lead to significant consequences, ranging from minor injuries to severe accidents with long-term health implications. Consider, for example, the potential dangers of handling corrosive chemicals. Without proper personal protective equipment (PPE) such as gloves and eye protection, even a small splash can cause severe burns or permanent eye damage. Similarly, improper handling of flammable materials can lead to fires or explosions, posing significant risks to personnel and property.
The practical significance of integrating safety practices into all laboratory procedures cannot be overstated. Safety protocols, including the use of fume hoods to prevent inhalation of toxic gases, the proper storage of chemicals to prevent unintended reactions, and the implementation of emergency procedures, are not mere formalities but essential safeguards. Regular safety training, drills, and inspections further reinforce a culture of safety, ensuring that all personnel are equipped with the knowledge and skills to respond effectively to potential hazards. For instance, understanding the appropriate fire extinguisher type for different classes of fires (e.g., Class A for ordinary combustibles, Class B for flammable liquids) can prevent a small incident from escalating into a major catastrophe. Similarly, knowing the proper procedure for dealing with a chemical spill, including containment and neutralization, can minimize environmental contamination and prevent potential exposure risks.
In conclusion, safety is not merely an adjunct to laboratory work but an inseparable element of its foundation. A robust safety culture, underpinned by comprehensive training, adherence to protocols, and a proactive approach to hazard identification and mitigation, is essential for ensuring a productive and secure research environment. The potential consequences of neglecting safety protocols underscore its critical role in any laboratory setting dedicated to investigating the physical and chemical properties of matter. Prioritizing safety not only protects personnel and property but also fosters an environment where scientific inquiry can flourish without unnecessary risks.
Frequently Asked Questions
This section addresses common inquiries regarding laboratory investigations of physical and chemical properties. Clarity on these points promotes effective experimentation and data interpretation.
Question 1: What is the difference between a physical and a chemical property?
A physical property can be observed or measured without changing the substance’s composition (e.g., density, melting point). A chemical property describes a substance’s potential to undergo a chemical change or reaction (e.g., flammability, reactivity with acids).
Question 2: Why is it important to maintain a controlled environment during experiments?
Environmental factors like temperature and pressure can significantly influence experimental outcomes. A controlled environment ensures consistency and allows for the isolation of specific variables, leading to more reliable and interpretable results.
Question 3: What are the potential hazards associated with a physical and chemical properties lab?
Hazards can include exposure to toxic or corrosive chemicals, flammable materials, sharp objects, and heat sources. Understanding these hazards and adhering to established safety protocols is crucial for preventing accidents.
Question 4: How can one ensure accurate measurements in the lab?
Accuracy relies on using properly calibrated instruments, following established measurement procedures, and minimizing potential sources of error. Repeating measurements and performing statistical analysis can further enhance accuracy.
Question 5: What is the importance of reproducibility in experimental work?
Reproducibility ensures that experimental findings are reliable and not due to chance or error. Documented procedures allow other researchers to replicate experiments and verify results, strengthening scientific conclusions.
Question 6: How does data analysis contribute to understanding material properties?
Data analysis transforms raw experimental data into meaningful information. Statistical analysis, graphical representation, and comparison with theoretical models help reveal trends, patterns, and relationships that elucidate material behavior.
Understanding these fundamental aspects of laboratory practice enhances experimental design, data interpretation, and overall scientific rigor. Accurate measurements, controlled environments, and a strong emphasis on safety are crucial for generating reliable and meaningful results in the investigation of material properties.
The subsequent sections will delve into specific experimental techniques and examples, building upon the foundational principles discussed here.
Conclusion
Exploration of material characteristics within a physical and chemical properties lab provides foundational knowledge essential for advancements across scientific disciplines. From fundamental concepts like density and melting point to complex chemical reactions and material interactions, rigorous investigation in controlled environments yields critical insights. Precise measurement, careful observation, and robust experimental design are cornerstones of this process, enabling accurate data analysis and interpretation. Emphasis on safety protocols ensures a secure environment for exploring the intricacies of matter.
Continued investigation within these specialized laboratory settings holds immense potential for future discoveries and innovations. Deepening understanding of material behavior paves the way for developing novel materials, optimizing existing technologies, and addressing complex scientific challenges. The pursuit of knowledge within the physical and chemical properties lab remains a vital driver of scientific and technological progress.