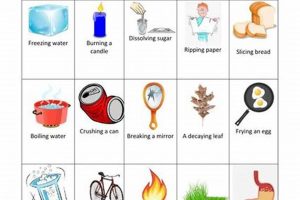
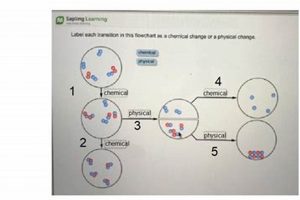
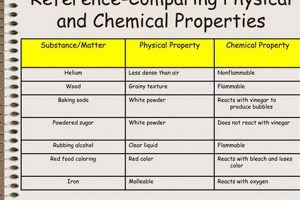
Experiments designed to differentiate between alterations in matter are foundational in scientific education. These investigations typically involve observing substances before, during, and after undergoing processes like melting, burning, dissolving, or reacting with other materials. For instance, observing the melting of ice demonstrates a shift in state without changing the underlying substance (water), while burning paper produces ash and gases, representing a fundamental change in composition.
Understanding the distinctions between these alterations is crucial for comprehending the behavior of matter in various contexts, from everyday occurrences to complex chemical reactions. Historically, the development of these concepts revolutionized fields like chemistry and materials science, enabling advancements in medicine, manufacturing, and environmental management. These investigations provide essential practical skills in observation, data collection, analysis, and critical thinking.
This foundation allows exploration of more complex concepts, including reaction rates, energy transfer, and the laws of thermodynamics. Further exploration may involve investigating specific types of reactions, such as acid-base reactions or oxidation-reduction reactions, deepening comprehension of chemical principles and their applications.
Tips for Effective Experimentation
Careful preparation and execution are essential for maximizing the educational value of investigative procedures involving material transformations.
Tip 1: Precise Observation: Record detailed observations of initial materials, including color, texture, and state. Note any changes during the process, such as color shifts, gas production, or temperature variations. Precise observations are crucial for accurate analysis and interpretation.
Tip 2: Controlled Experiments: When comparing different processes, ensure all variables except the independent variable are controlled. This isolates the impact of the specific change being studied. For example, when comparing the melting rates of different substances, use the same heat source and initial temperature.
Tip 3: Safety Precautions: Adhere to established safety protocols. Wear appropriate protective equipment, including goggles and gloves. Handle chemicals with care and dispose of materials responsibly according to guidelines.
Tip 4: Accurate Measurements: Use appropriate instruments for accurate measurements of mass, volume, and temperature. Proper calibration and technique minimize errors and enhance the reliability of results.
Tip 5: Data Recording: Organize data systematically in tables or charts. This facilitates analysis and identification of trends or patterns. Include units of measurement and significant figures for clarity.
Tip 6: Interpretation and Analysis: Connect observations and data to underlying scientific principles. Distinguish between qualitative and quantitative data and explain the observed changes in terms of molecular interactions.
Tip 7: Thorough Documentation: Maintain detailed records of procedures, observations, data, and analysis. Clear documentation ensures reproducibility and supports the validity of conclusions.
Careful attention to these guidelines ensures robust experimental design, leading to a deeper understanding of the nature and implications of material alterations.
By following these tips, one can gain a comprehensive understanding of the principles governing transformations of matter and their significance in various scientific disciplines.
1. Observation
Observation forms the cornerstone of scientific inquiry, particularly within investigations involving material transformations. Accurate and detailed observation provides the foundational data upon which analysis, interpretation, and conclusions are built. It serves as the bridge between the physical phenomena occurring within an experiment and the scientific understanding derived from it.
- Qualitative Observations:
Qualitative observations focus on descriptive qualities. Examples include noting color changes (e.g., a solution turning blue), changes in state (e.g., a solid melting into a liquid), or the formation of a precipitate. In the context of differentiating between physical and chemical changes, qualitative observations provide essential clues. For example, observing a change in color upon mixing two solutions may suggest a chemical reaction, while observing a change in state upon heating suggests a physical change.
- Quantitative Observations:
Quantitative observations involve numerical measurements. These include measuring temperature changes (e.g., a 5C increase), mass changes (e.g., a 2-gram decrease), or volume changes (e.g., a 10 mL increase). Quantitative data provides precise evidence for analysis. For example, measuring the mass of reactants and products in a chemical reaction allows for the determination of mass conservation.
- Systematic Recording:
Systematic recording of observations is crucial for accurate interpretation. Data should be recorded in a structured format, such as tables or graphs, with clear labels and units. This structured approach facilitates identification of trends and patterns. For instance, plotting temperature changes over time during a reaction can reveal the exothermic or endothermic nature of the process.
- Objective Observation:
Objective observation requires avoiding personal bias or preconceived notions. Observations should be based solely on the empirical evidence gathered during the experiment. Objectivity ensures the reliability and validity of the results. For example, avoiding subjective descriptions like “the reaction looked interesting” and focusing on specific changes, like “the solution effervesced vigorously,” ensures the data remains unbiased.
The interplay between qualitative and quantitative observations, coupled with meticulous recording and a commitment to objectivity, allows for robust analysis of experimental data. This comprehensive approach to observation provides a strong foundation for understanding the nature and characteristics of both physical and chemical changes within laboratory settings.
2. Experimentation
Experimentation serves as the active component within investigations of material transformations, providing a structured framework for manipulating variables and observing outcomes. It allows for the controlled testing of hypotheses related to physical and chemical changes, enabling researchers to distinguish between processes that alter form and those that alter composition. A classic example involves heating a sugar sample. Observation alone might suggest melting, a physical change. However, continued heating leads to caramelization and eventual burning, demonstrating a chemical change through color alteration and the release of gases. Controlled experiments, varying temperature and exposure to air, allow for isolation of these distinct processes and the determination of conditions influencing each.
The core of experimentation lies in the manipulation of independent variables and the measurement of dependent variables. In investigating reaction rates, varying reactant concentrations (independent variable) while measuring the rate of product formation (dependent variable) exemplifies this process. Such controlled manipulation allows for the establishment of cause-and-effect relationships, deepening understanding of the factors influencing transformations. Moreover, experimentation facilitates the replication of phenomena, allowing for the validation of results and the refinement of scientific models. Reproducibility strengthens the reliability and generalizability of findings related to physical and chemical changes.
Experimentation within the context of material transformation studies plays a vital role in both fundamental research and practical applications. Developing new materials relies heavily on understanding how processing conditions influence material properties, which is driven by experimentation. Similarly, forensic science employs experimentation to analyze evidence and reconstruct events, often by distinguishing between physical traces and chemical reactions. Challenges such as controlling all variables and ensuring accurate measurements require careful experimental design and execution. Overcoming these challenges strengthens scientific rigor and enhances understanding of the complex interplay of factors governing material changes.
3. Analysis
Analysis forms an essential bridge between observation and understanding within the context of a physical and chemical change laboratory. It involves systematically examining collected databoth qualitative and quantitativeto identify trends, patterns, and relationships that reveal the underlying nature of the transformations observed. For instance, if a gas is evolved during a reaction and identified as carbon dioxide through further testing, analysis connects this observation to a potential chemical change involving the decomposition of a carbonate. Similarly, a consistent mass change following the evaporation of a solvent confirms the physical nature of the transformation, aligning with the principle of conservation of mass. Analysis is not merely about summarizing results; it’s about extracting meaning from the data and connecting empirical observations to theoretical frameworks.
Effective analysis requires careful consideration of the experimental design. Understanding potential sources of error, such as limitations in measurement precision or uncontrolled variables, is crucial for interpreting the significance of the results. Consider an experiment comparing the reactivity of different metals with an acid. If the metal samples have varying surface areas, this factor could confound the interpretation of reaction rates, potentially masking the true impact of the metal’s inherent reactivity. Therefore, meticulous experimental design and rigorous data analysis must work in concert to ensure valid conclusions. Furthermore, analysis often involves constructing graphical representations of data, performing statistical tests, or applying mathematical models to quantify relationships and draw robust conclusions. For example, plotting the volume of gas produced over time can reveal the order of a reaction, offering deeper insights into the reaction mechanism.
The practical significance of analysis within this context extends beyond the laboratory. Understanding material transformations is crucial in diverse fields. In materials science, analyzing the impact of heat treatments on metal alloys informs the development of stronger and more durable materials. Environmental scientists analyze the composition of pollutants to understand their sources and environmental impacts. In medicine, analyzing blood samples for specific markers helps diagnose and monitor diseases. The analytical skills honed in a physical and chemical change laboratory are thus transferable and highly valuable in a broad range of scientific and technological applications. The challenge lies in developing the ability to critically evaluate data, recognizing limitations, and formulating sound judgments based on evidence, skills crucial for advancing scientific knowledge and its practical applications.
4. Interpretation
Interpretation within the context of a physical and chemical change laboratory signifies the process of assigning meaning to observed phenomena and experimental data. It involves connecting empirical evidence with established scientific principles to draw conclusions about the nature of the transformations under investigation. Accurate interpretation is essential for distinguishing between physical changes, which alter form without affecting composition, and chemical changes, which result in new substances with different properties. This process often requires careful consideration of multiple factors and the integration of various pieces of evidence to arrive at a comprehensive understanding of the observed changes.
- Connecting Observations to Theory
Interpretation bridges the gap between raw experimental data and established scientific theories. For example, observing the formation of a precipitate upon mixing two clear solutions could be interpreted as evidence of a chemical reaction forming an insoluble product. This interpretation links the observation to the theoretical concept of solubility and chemical reactivity. Similarly, observing a change in state without a change in composition, such as the melting of ice, can be interpreted as a physical change based on the understanding of phase transitions and intermolecular forces.
- Explaining Discrepancies and Anomalies
Interpretation involves not only explaining expected results but also addressing discrepancies and anomalies. If a reaction produces less product than predicted by stoichiometry, interpretation requires considering potential sources of error, such as incomplete reactions, side reactions, or measurement inaccuracies. This critical analysis of discrepancies is vital for refining experimental procedures and deepening understanding of the underlying chemical processes. For instance, an unexpected color change during a reaction might indicate the presence of an impurity or the occurrence of an unforeseen side reaction, prompting further investigation.
- Drawing Conclusions and Making Predictions
Interpretation culminates in drawing conclusions based on the available evidence and making predictions about future behavior. If a substance is identified as an acid based on its reaction with a base, one could predict its behavior in other chemical contexts, such as its reaction with a metal. These predictions can then be tested through further experimentation, creating a cycle of observation, experimentation, analysis, and interpretation that drives scientific discovery. For example, understanding the factors affecting reaction rates allows for predictions about how changing temperature or concentration will influence the speed of a chemical transformation.
- Communicating Findings Effectively
Interpretation also involves communicating findings effectively to others. Clear and concise explanations, supported by evidence and logical reasoning, are crucial for disseminating scientific knowledge. This communication might involve writing laboratory reports, presenting research findings at conferences, or explaining scientific concepts to a broader audience. The ability to articulate the meaning and implications of experimental results is essential for advancing scientific understanding and fostering collaboration among researchers. Effectively conveying the distinction between physical and chemical changes, for example, requires clear articulation of the observed changes and the underlying scientific principles supporting the interpretation.
These interconnected facets of interpretation are essential for extracting meaningful insights from the data generated within a physical and chemical change laboratory. The process of interpreting experimental results not only strengthens understanding of the specific transformations under investigation but also cultivates critical thinking skills and promotes deeper engagement with scientific principles, fostering a more nuanced understanding of the behavior of matter.
5. Documentation
Thorough documentation forms an integral component of investigations involving physical and chemical changes within laboratory settings. Meticulous record-keeping ensures data integrity, facilitates result reproducibility, and enables effective communication of findings. Documentation encompasses all aspects of the experimental process, from initial experimental design and material preparation to observations, data collection, analysis, and interpretation. A well-maintained laboratory notebook serves as the primary tool for documentation, providing a chronological record of experimental activities. Consider a scenario involving the synthesis of a new compound. Detailed documentation of the reactants used, their quantities, reaction conditions (temperature, pressure, time), and observed changes (color change, gas evolution) are crucial for reproducing the synthesis and verifying the results. Furthermore, any deviations from the planned procedure, unexpected observations, or potential sources of error must be carefully documented. This level of detail enables critical evaluation of the experimental results and facilitates troubleshooting if discrepancies arise.
The importance of documentation extends beyond individual experiments. In collaborative research environments, clear and comprehensive documentation allows other researchers to understand, replicate, and build upon previous work. This fosters transparency and accelerates scientific progress. For instance, in pharmaceutical research, detailed documentation of drug synthesis and testing procedures is essential for regulatory approval and ensures the safety and efficacy of new medications. Moreover, documentation plays a crucial role in intellectual property protection and establishing precedence in scientific discoveries. A complete and accurate record of experimental procedures and results can serve as vital evidence in patent applications or scientific disputes. The practical significance of proper documentation is evident in fields such as forensic science, where meticulous record-keeping is essential for maintaining chain of custody and ensuring the admissibility of evidence in legal proceedings.
In summary, meticulous documentation is not merely a bureaucratic requirement but a fundamental aspect of scientific practice, particularly in studies involving physical and chemical changes. It underpins the integrity, reproducibility, and communicability of scientific findings. While the specific requirements for documentation may vary across disciplines and research settings, the underlying principle remains consistent: a comprehensive and accurate record of experimental work is essential for advancing scientific knowledge and ensuring its responsible application. Challenges such as data management and ensuring data security necessitate robust documentation practices. Addressing these challenges reinforces the value of meticulous record-keeping in upholding the highest standards of scientific rigor and integrity within the broader scientific community.
6. Safety
Safety within a physical and chemical change laboratory is paramount. The inherent nature of these investigations involves potential hazards, necessitating rigorous safety protocols to minimize risks and ensure a secure working environment. Experiments often involve handling chemicals, using heat sources, and generating potentially hazardous byproducts. A seemingly simple procedure, such as dissolving a metal in acid, can generate flammable hydrogen gas, illustrating the potential for unexpected hazards. Therefore, understanding the specific hazards associated with each experiment and implementing appropriate safety measures are crucial for preventing accidents and ensuring the well-being of all laboratory personnel. Cause-and-effect relationships between experimental procedures and potential hazards must be clearly understood. For example, heating flammable liquids without proper ventilation can lead to fire or explosions. This understanding underscores the importance of risk assessment before commencing any experiment.
Practical examples highlight the significance of safety protocols. Wearing appropriate personal protective equipment (PPE), such as safety goggles, gloves, and lab coats, provides a barrier against chemical splashes, burns, and cuts. Proper ventilation systems remove potentially harmful vapors and gases, preventing inhalation or exposure. Additionally, knowing the location and proper use of safety equipment, such as fire extinguishers, eye wash stations, and safety showers, is essential for responding effectively to emergencies. Regular inspection and maintenance of equipment further minimize risks. For instance, ensuring that gas lines are properly sealed and electrical equipment is functioning correctly can prevent leaks and fires. Furthermore, adherence to established chemical handling and disposal procedures prevents environmental contamination and minimizes long-term health risks. Proper labeling of chemicals, along with maintaining up-to-date safety data sheets (SDS), provides crucial information about hazards and appropriate handling procedures.
Challenges in maintaining laboratory safety include complacency and inadequate training. Regular safety training and reinforcement of best practices are crucial for fostering a safety-conscious culture. Addressing these challenges strengthens the overall safety framework and minimizes the likelihood of accidents. The practical significance of prioritizing safety extends beyond preventing immediate harm. It fosters a positive learning environment where individuals can engage with scientific investigations confidently and responsibly, promoting both scientific advancement and personal well-being. Ultimately, a commitment to safety reflects a commitment to ethical and responsible scientific practice.
Frequently Asked Questions
Addressing common inquiries regarding the differentiation between physical and chemical transformations in laboratory settings is crucial for fostering a comprehensive understanding of these fundamental concepts.
Question 1: How can one definitively determine if a change is physical or chemical?
A chemical change results in the formation of new substances with different properties, often evidenced by color changes, gas production, precipitate formation, or significant temperature changes. Physical changes alter a substance’s form without changing its composition, such as changes in state (solid, liquid, gas) or shape.
Question 2: Is dissolving a substance in water a physical or chemical change?
Dissolving can be either. When sugar dissolves in water, it undergoes a physical change; the sugar molecules disperse but retain their chemical identity. Dissolving a metal in acid, however, involves a chemical reaction, producing new substances.
Question 3: Can physical changes be reversed? What about chemical changes?
Physical changes are generally easily reversed, as the original substance remains. Melting ice can be reversed by freezing. Chemical changes are typically more difficult to reverse, as new substances are formed. While some reactions are reversible, they often require specific conditions.
Question 4: Why is understanding the difference between these changes important?
Distinguishing between these transformations is fundamental to comprehending material behavior. This understanding is crucial in fields like materials science, chemical engineering, and environmental science, informing material design, process optimization, and environmental management.
Question 5: Are all chemical changes accompanied by visible changes?
Not all chemical changes are readily apparent. Some reactions may occur without noticeable changes in color, temperature, or state. Sophisticated analytical techniques may be required to confirm their occurrence.
Question 6: How does laboratory experimentation facilitate understanding of these changes?
Laboratory investigations allow for controlled manipulation of variables and systematic observation of changes in matter. This controlled environment enables researchers to isolate specific transformations, collect quantitative data, and draw conclusions about the nature of the observed changes.
Careful observation, rigorous experimentation, and accurate analysis are essential for differentiating between physical and chemical transformations. Understanding these fundamental concepts is crucial for advancing scientific knowledge and its applications across diverse fields.
This foundational understanding prepares for exploration of more advanced concepts related to chemical reactions, stoichiometry, and thermodynamics.
Conclusion
Investigations into the nature of physical and chemical changes within laboratory settings provide essential foundations for scientific understanding. Careful observation, rigorous experimentation, and detailed analysis enable differentiation between transformations that alter form and those that alter composition. From systematic documentation practices to paramount safety considerations, adhering to established protocols ensures both the integrity of experimental results and the well-being of laboratory personnel. Addressing common inquiries and misconceptions further strengthens comprehension of these fundamental concepts and their broad implications.
Continued exploration and refinement of experimental techniques promise deeper insights into the intricate mechanisms governing material transformations. Such advancements hold profound implications for diverse fields, ranging from materials science and chemical engineering to environmental management and medicine. Cultivating a robust understanding of physical and chemical changes empowers informed decision-making and fosters innovation across scientific disciplines, propelling further exploration and discovery.