Laboratory exercises focusing on differentiating between substance characteristics often involve observations and measurements related to changes in matter. For example, a student might record the melting point of a solid or observe the reaction of a metal with an acid. These exercises typically require documenting observations and providing explanations based on scientific principles. Data collected might include quantitative measurements like mass, volume, and temperature, as well as qualitative observations like color change, gas evolution, or precipitate formation. Analysis often involves classifying observed characteristics as either intensive or extensive and interpreting results in the context of atomic and molecular interactions.
Understanding the distinction between intrinsic and extrinsic properties of matter is fundamental to numerous scientific disciplines, including chemistry, materials science, and engineering. This knowledge is crucial for designing new materials, predicting the behavior of substances under various conditions, and interpreting chemical reactions. Historically, the development of this understanding has been instrumental in advancing fields from metallurgy and alchemy to modern chemical synthesis and pharmaceutical development. Accurate observation and meticulous record-keeping in laboratory settings have been essential for building this knowledge base over centuries.
This foundational understanding of matter provides a basis for exploring more complex concepts like chemical bonding, thermodynamics, and reaction kinetics. Further investigation might involve more advanced laboratory techniques, computational modeling, and theoretical analysis.
Tips for Analyzing Physical and Chemical Properties in Lab Settings
Accurate analysis of experimental data is crucial for drawing valid conclusions in scientific investigations. The following tips offer guidance for effective interpretation of observations and measurements related to material properties.
Tip 1: Precise Measurement: Ensure accurate data collection by using appropriate instruments and techniques. Calibrate instruments regularly and record measurements with the correct number of significant figures. For example, when measuring volume, select a graduated cylinder with appropriate graduations for the desired precision.
Tip 2: Detailed Observation: Document all observations thoroughly, including both qualitative and quantitative data. Note any changes in color, state, temperature, or other relevant properties. For example, describe a precipitate as “a white, crystalline solid” rather than simply “a precipitate.”
Tip 3: Control Experiments: Use control experiments to isolate the effects of specific variables. A control provides a baseline for comparison, allowing for more confident attribution of observed changes to the independent variable.
Tip 4: Data Organization: Organize data systematically using tables and graphs. This facilitates analysis and identification of trends or patterns. Clearly label axes and include units of measurement.
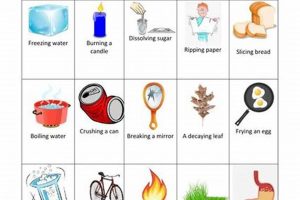
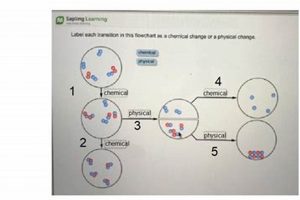
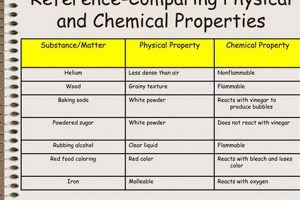
Tip 5: Property Differentiation: Clearly distinguish between physical and chemical properties. Physical properties can be observed without changing the substance’s identity, while chemical properties involve changes in composition. For instance, density is a physical property, while flammability is a chemical property.
Tip 6: Error Analysis: Acknowledge potential sources of error and their impact on results. Discuss both systematic and random errors and suggest ways to minimize their influence in future experiments.
By adhering to these guidelines, experimental outcomes can be interpreted with greater confidence, contributing to a more robust understanding of the underlying scientific principles.
These practical laboratory techniques are essential for any scientific investigation involving the characterization of matter, laying the groundwork for more advanced studies and applications.
1. Observation
Accurate observation forms the bedrock of scientific inquiry, especially in laboratory settings investigating physical and chemical properties. Rigorous observation provides the initial data upon which subsequent analyses and interpretations are built. Without careful attention to detail, crucial information can be missed, leading to incomplete or inaccurate conclusions.
- Qualitative Observation
Qualitative observation involves describing characteristics using sensory information, such as color, odor, texture, or state of matter. For example, noting the formation of a precipitate as cloudy white or describing a reaction as vigorous bubbling provides valuable qualitative data. These descriptions, while not numerical, contribute significantly to understanding the nature of the substances and their interactions.
- Quantitative Observation
Quantitative observation focuses on measurable properties using instruments. Recording numerical data, such as temperature change, mass, volume, or pH, provides precise information for analysis. For example, measuring the melting point of a substance provides a specific value crucial for identification and characterization. The precision of quantitative data is essential for accurate interpretations and comparisons.
- Systematic Observation
Systematic observation involves following a structured approach to data collection, ensuring consistency and reducing bias. This might involve using a standardized checklist or following a specific experimental protocol. Systematic observation ensures all relevant aspects are considered and documented, contributing to the reliability and reproducibility of experimental results.
- Inferential Observation
Inferential observation involves drawing preliminary conclusions based on observed data. While not definitive, inferences provide a starting point for formulating hypotheses and guiding further investigation. For example, observing a gas being evolved during a reaction might lead to an inference about the production of a gaseous product. These inferences must be tested through further experimentation and analysis.
These interconnected facets of observation are essential for constructing a comprehensive understanding of material properties in a laboratory setting. The interplay of qualitative and quantitative data, gathered through systematic observation and leading to initial inferences, sets the stage for rigorous scientific analysis. By prioritizing meticulous observation, researchers ensure a solid foundation for drawing valid conclusions and advancing scientific knowledge. This observational foundation is essential for accurately determining whether observed changes represent physical alterations or transformations at the chemical level.
2. Measurement
Measurement plays a crucial role in determining physical and chemical properties within a laboratory setting. Quantitative data acquired through measurement provides the basis for distinguishing between different substances and understanding their behavior. Precise measurements are essential for accurate identification, classification, and interpretation of material properties. For instance, determining the density of a substance requires precise measurements of mass and volume. Melting point determination relies on accurate temperature measurement. Similarly, assessing the acidity or alkalinity of a solution requires precise pH measurements. These measurements provide essential information for characterizing materials and predicting their interactions.
The relationship between measurement and material properties extends beyond simple identification. Changes in measured properties can indicate chemical reactions or physical transformations. For example, observing a change in temperature during a reaction can signify an exothermic or endothermic process. A change in mass can indicate gas evolution or consumption. Precise measurement of these changes allows for quantification of reaction rates and thermodynamic parameters. Furthermore, accurate measurements are critical for establishing relationships between different properties, such as the relationship between temperature and pressure for a gas, or the relationship between concentration and reaction rate. These relationships, often expressed mathematically, provide deeper insights into the behavior of matter.
Challenges in measurement can arise from limitations in instrument precision, experimental error, and the inherent variability of some properties. Addressing these challenges requires careful experimental design, appropriate instrument calibration, and statistical analysis of data. Overcoming these challenges allows for reliable determination of material properties and a more robust understanding of the underlying scientific principles. Accurate measurement, therefore, is not merely a procedural step but a foundational element in scientific investigations, enabling a deeper understanding of the physical and chemical world. It allows for the precise characterization of materials, the quantification of chemical and physical changes, and the development of predictive models based on established relationships between different properties.
3. Identification
Substance identification is integral to analyzing physical and chemical properties in laboratory settings. Accurate identification provides context for interpreting observed characteristics and behaviors. Without correct identification, experimental results lack meaning and cannot be reliably applied to broader scientific understanding. This process often involves comparing measured properties against known values and employing systematic analytical techniques. The ability to identify substances correctly is crucial for drawing valid conclusions from experimental data.
- Reference Materials
Comparing measured properties to established reference values is a primary method for identification. Resources like chemical handbooks and online databases provide comprehensive data on known substances. Matching observed melting points, boiling points, densities, and other characteristic properties to these reference values enables researchers to identify unknown materials. Discrepancies between measured and reference values can indicate impurities or errors in measurement, highlighting the importance of accuracy and precision in laboratory procedures. For example, an unknown liquid with a boiling point of 100C and a density of 1 g/mL strongly suggests water.
- Spectroscopic Techniques
Spectroscopic methods analyze the interaction of matter with electromagnetic radiation to identify substances. Techniques like infrared (IR) spectroscopy and nuclear magnetic resonance (NMR) spectroscopy provide unique fingerprints of molecules based on their vibrational and nuclear spin properties, respectively. These spectral fingerprints can be compared to libraries of known spectra to identify the composition of unknown samples. For instance, the presence of specific functional groups in an organic molecule can be determined using IR spectroscopy.
- Chemical Tests
Chemical tests involve observing reactions with specific reagents to determine the presence or absence of particular functional groups or elements. These tests often produce characteristic color changes, precipitate formation, or gas evolution. For example, the addition of silver nitrate to a solution can confirm the presence of chloride ions by producing a white precipitate of silver chloride. Similarly, flame tests can identify metal ions based on the characteristic color they impart to a flame. These tests rely on the unique chemical properties of substances to provide qualitative identification.
- Chromatographic Methods
Chromatographic techniques separate mixtures into their components based on their differential interaction with a stationary and mobile phase. This separation allows for the identification of individual components by comparing their retention times or other characteristics to known standards. For instance, gas chromatography coupled with mass spectrometry (GC-MS) can identify the individual components in a complex mixture of volatile organic compounds.
The accurate identification of substances is essential for interpreting the results of experiments involving physical and chemical properties. Whether through comparison with reference data, spectroscopic analysis, chemical tests, or chromatographic separation, proper identification provides the framework for understanding observed behaviors and drawing meaningful conclusions. This process of identification links laboratory observations to the broader body of scientific knowledge, facilitating the advancement of understanding in various fields from materials science to environmental analysis.
4. Classification
Classification is fundamental to organizing and interpreting data related to physical and chemical properties in a laboratory setting. Distinguishing between different types of properties allows for systematic analysis and prediction of substance behavior. Correct classification provides a framework for understanding the nature of matter and its interactions.
- Physical Properties
Physical properties describe characteristics that can be observed or measured without altering the substance’s chemical composition. Examples include color, density, melting point, boiling point, and hardness. In a laboratory context, these properties are often used for initial characterization and identification of substances. Changes in physical properties, such as a phase transition from solid to liquid, provide insights into the energy changes associated with physical transformations. Understanding physical properties is essential for predicting how a substance will behave under different conditions without undergoing a chemical reaction.
- Chemical Properties
Chemical properties describe a substance’s potential to undergo chemical change, resulting in the formation of new substances with different compositions. Flammability, reactivity with acids or bases, and oxidation potential are examples of chemical properties. Laboratory experiments often explore these properties through controlled reactions to determine how a substance interacts with other chemicals. Observing changes like gas evolution, precipitate formation, or color change during these reactions provides evidence of chemical transformations. Understanding chemical properties is crucial for predicting how a substance will react in different chemical environments and for synthesizing new materials.
- Intensive Properties
Intensive properties are independent of the amount of substance present. Temperature, density, and color are examples. These properties are particularly useful for substance identification as they remain constant regardless of sample size. In a lab setting, measuring intensive properties allows for comparison between different samples of the same substance or for distinguishing between different substances altogether, regardless of the quantity being analyzed.
- Extensive Properties
Extensive properties depend on the amount of substance. Mass, volume, and energy are examples. These properties are often measured in laboratory experiments to quantify the amount of material involved in a reaction or process. Changes in extensive properties, like a change in mass during a chemical reaction, provide quantitative data for stoichiometric calculations and determining reaction yields. While not useful for identification, extensive properties are crucial for understanding the scale of a process or reaction.
The classification of properties as physical or chemical, intensive or extensive, is essential for organizing experimental data and drawing meaningful conclusions in a laboratory setting. This classification scheme provides a framework for understanding the diverse characteristics of matter and predicting its behavior under various conditions. By systematically classifying observed properties, researchers can gain a deeper understanding of the fundamental principles governing the physical and chemical world.
5. Interpretation
Interpretation is the crucial final step in analyzing physical and chemical properties within a laboratory setting. Raw data, whether qualitative observations or quantitative measurements, holds limited value without thoughtful interpretation. This process involves connecting observed phenomena to underlying scientific principles, establishing cause-and-effect relationships, and drawing meaningful conclusions. Interpretation transforms data into understanding, enabling researchers to explain observed behaviors and predict future outcomes. For instance, observing a vigorous reaction between a metal and an acid, coupled with the measurement of hydrogen gas evolution, might be interpreted as evidence of an acid-metal reaction producing a metal salt and hydrogen gas. This interpretation relies on understanding the chemical properties of acids and metals and the principles of chemical reactions. The practical significance of such interpretation lies in its ability to predict the outcome of similar reactions and to apply this understanding to real-world applications, such as corrosion prevention.
Accurate interpretation requires a strong foundation in scientific concepts and principles. It involves considering potential sources of error, evaluating the validity of assumptions, and critically analyzing the data. For example, a measured melting point lower than the expected value might be interpreted as the presence of impurities in the sample. This interpretation requires understanding the effect of impurities on colligative properties. Furthermore, interpretation often involves integrating multiple pieces of evidence. Combining observations of a color change with spectroscopic data might lead to the identification of a specific chemical compound formed during a reaction. This integration of different data types strengthens the interpretation and provides a more comprehensive understanding of the chemical process.
The ability to interpret experimental data accurately is essential for advancing scientific knowledge and solving real-world problems. It bridges the gap between observation and understanding, enabling researchers to explain observed phenomena and develop predictive models. Challenges in interpretation can arise from incomplete data, experimental error, or limitations in current scientific understanding. Addressing these challenges requires rigorous experimental design, careful data analysis, and a willingness to revise interpretations as new evidence emerges. Ultimately, the process of interpretation transforms laboratory observations into meaningful insights, contributing to a deeper understanding of the physical and chemical world and its practical applications.
Frequently Asked Questions
This section addresses common queries regarding the analysis and interpretation of physical and chemical properties in laboratory investigations.
Question 1: How does one differentiate between a physical and a chemical change?
A physical change alters a substance’s form without affecting its chemical composition, such as melting ice. A chemical change involves the formation of new substances with different compositions, exemplified by the rusting of iron.
Question 2: What is the significance of significant figures in property measurements?
Significant figures reflect the precision of a measurement. Recording and reporting the correct number of significant figures ensures accurate representation of the measurement’s uncertainty and facilitates meaningful comparisons between different measurements.
Question 3: Why are control experiments essential in investigations of material properties?
Control experiments provide a baseline for comparison, allowing researchers to isolate the effects of specific variables and attribute observed changes to the independent variable with greater confidence.
Question 4: How can spectroscopic techniques assist in identifying unknown substances?
Spectroscopic methods, like IR and NMR spectroscopy, generate unique spectral fingerprints of molecules based on their interaction with electromagnetic radiation. Comparing these fingerprints to established spectral libraries allows for identification of unknown compounds.
Question 5: What are the limitations of relying solely on physical properties for substance identification?
While physical properties offer valuable clues, relying solely on them can be insufficient for definitive identification, especially for isomers or complex mixtures. Combining physical property analysis with other techniques, such as chemical tests or spectroscopic analysis, enhances identification accuracy.
Question 6: How does understanding material properties contribute to real-world applications?
Knowledge of material properties is fundamental to various fields, including materials science, engineering, and medicine. It informs the design of new materials with specific properties, predicts the behavior of materials under various conditions, and facilitates the development of new technologies and medical treatments.
Addressing these frequently asked questions strengthens one’s understanding of key concepts related to physical and chemical property analysis. This knowledge forms the basis for robust experimental design, accurate data interpretation, and meaningful contributions to scientific inquiry.
Further exploration of specific laboratory techniques and their applications can provide a deeper understanding of material characterization methodologies.
Conclusion
Accurate determination of physical and chemical properties through laboratory investigation is fundamental to understanding the nature and behavior of matter. Systematic observation, precise measurement, and correct identification are crucial prerequisites for classifying and interpreting experimental data. Distinguishing between physical and chemical changes, recognizing the significance of intensive and extensive properties, and utilizing analytical techniques like spectroscopy and chromatography are essential for robust analysis. Furthermore, careful interpretation of results, informed by scientific principles and an awareness of potential errors, allows for drawing valid conclusions and connecting experimental findings to broader scientific understanding.
Continued advancements in analytical techniques and a deeper understanding of the underlying principles governing material behavior will further enhance the ability to characterize and manipulate matter. This ongoing pursuit of knowledge has profound implications for various scientific disciplines and technological advancements, paving the way for new discoveries and innovations that shape our understanding of the physical world.